
$S{{i}_{2}}O_{7}^{-6}$ anion is obtained when:
A) no oxygen of a $Si{{O}_{4}}$ tetrahedron is shared with another $Si{{O}_{4}}$ tetrahedron
B) one oxygen of a $Si{{O}_{4}}$ tetrahedron is shared with another $Si{{O}_{4}}$ tetrahedron
C) two oxygen of a $Si{{O}_{4}}$ tetrahedron is shared with another $Si{{O}_{4}}$ tetrahedron
D) three or all four oxygen of a $Si{{O}_{4}}$ tetrahedron is shared with another $Si{{O}_{4}}$ tetrahedron
Answer
558.9k+ views
Hint: Anions are defined as the ions that have the tendency to gain electrons. It consists of negative charge. For example, bromide, chloride, cyanide. Anions are of different types: monovalent, bivalent.
Complete answer:
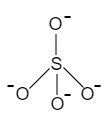
First let us see the structure of ${{\left[ Si{{O}_{4}} \right]}^{4-}}$
Let us discuss all the given options one by one:
When no oxygen of a $Si{{O}_{4}}$ tetrahedron is shared with another $Si{{O}_{4}}$ tetrahedron, it only gives $Si{{O}_{4}}$ tetrahedron, which is known as, orthosilicates.
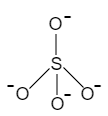
For example, $Z{{n}_{2}}Si{{O}_{4}},\,\,M{{g}_{2}}Si{{O}_{4}}$
Therefore, option (A) is incorrect.
When one oxygen of a $Si{{O}_{4}}$ tetrahedron is shared with another $Si{{O}_{4}}$ tetrahedron, it results in the formation of pyro silicates. Let us see how pyro silicate is formed.
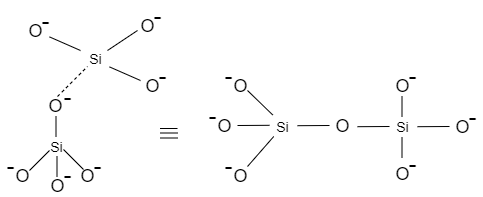
This structure is known as pyro silicate having a formula of $S{{i}_{2}}O_{7}^{-6}$ .
Therefore, this option is correct.
When two oxygen of a $Si{{O}_{4}}$ tetrahedron is shared with another $Si{{O}_{4}}$ tetrahedron, and forms closed rings, then it results in the formation of cyclic silicates. The general formula of cyclic silicate is ${{\left( SiO_{3}^{2-} \right)}_{n}}$ where $n$ is the number of tetrahedral units to form a ring.
When $n$ is equal to $3$
General formula: ${{\left( SiO_{3}^{2-} \right)}_{n}}$
Now, substituting the value of $n$ in the above formula, we get,
${{\left( SiO_{3}^{2-} \right)}_{3}}$
$\Rightarrow S{{i}_{3}}O_{9}^{-6}$ ion
Let us see the structure.
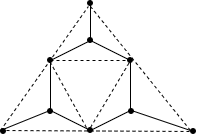
This structure is known as wollastonite. Therefore, this option is also incorrect.
When three or all four oxygen of a $Si{{O}_{4}}$ tetrahedron is shared with another $Si{{O}_{4}}$ tetrahedron, it results in the formation of sheet silicate or phyllosilicate.
The general formula of sheet silicate is: $\left( S{{i}_{2}}{{O}_{5}} \right)_{n}^{-2n}$
If we use the value of $n=1,2,3,4,5$ then also we will not get the formula $S{{i}_{2}}O_{7}^{-6}$
Therefore, this option is also incorrect.
Hence,the correct option is B.
Note:In this question, we have discussed different types of silicates, that are, ortho silicate, pyro silicate, cyclic silicate and sheet silicate.Silicates are defined as the compounds that consist of silicon and oxygen atoms.In sheet silicate, only one oxygen atom is left, $Si{{O}_{4}}$ tetrahedron. One unit negative charge is left which gets neutralized by cations as cations contain positive charge.
Complete answer:
First let us see the structure of ${{\left[ Si{{O}_{4}} \right]}^{4-}}$

Let us discuss all the given options one by one:
When no oxygen of a $Si{{O}_{4}}$ tetrahedron is shared with another $Si{{O}_{4}}$ tetrahedron, it only gives $Si{{O}_{4}}$ tetrahedron, which is known as, orthosilicates.

For example, $Z{{n}_{2}}Si{{O}_{4}},\,\,M{{g}_{2}}Si{{O}_{4}}$
Therefore, option (A) is incorrect.
When one oxygen of a $Si{{O}_{4}}$ tetrahedron is shared with another $Si{{O}_{4}}$ tetrahedron, it results in the formation of pyro silicates. Let us see how pyro silicate is formed.

This structure is known as pyro silicate having a formula of $S{{i}_{2}}O_{7}^{-6}$ .
Therefore, this option is correct.
When two oxygen of a $Si{{O}_{4}}$ tetrahedron is shared with another $Si{{O}_{4}}$ tetrahedron, and forms closed rings, then it results in the formation of cyclic silicates. The general formula of cyclic silicate is ${{\left( SiO_{3}^{2-} \right)}_{n}}$ where $n$ is the number of tetrahedral units to form a ring.
When $n$ is equal to $3$
General formula: ${{\left( SiO_{3}^{2-} \right)}_{n}}$
Now, substituting the value of $n$ in the above formula, we get,
${{\left( SiO_{3}^{2-} \right)}_{3}}$
$\Rightarrow S{{i}_{3}}O_{9}^{-6}$ ion
Let us see the structure.

This structure is known as wollastonite. Therefore, this option is also incorrect.
When three or all four oxygen of a $Si{{O}_{4}}$ tetrahedron is shared with another $Si{{O}_{4}}$ tetrahedron, it results in the formation of sheet silicate or phyllosilicate.
The general formula of sheet silicate is: $\left( S{{i}_{2}}{{O}_{5}} \right)_{n}^{-2n}$
If we use the value of $n=1,2,3,4,5$ then also we will not get the formula $S{{i}_{2}}O_{7}^{-6}$
Therefore, this option is also incorrect.
Hence,the correct option is B.
Note:In this question, we have discussed different types of silicates, that are, ortho silicate, pyro silicate, cyclic silicate and sheet silicate.Silicates are defined as the compounds that consist of silicon and oxygen atoms.In sheet silicate, only one oxygen atom is left, $Si{{O}_{4}}$ tetrahedron. One unit negative charge is left which gets neutralized by cations as cations contain positive charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




