
Show the formation of \[N{{a}_{2}}O\] and MgO by transfer of electrons.
Answer
589.5k+ views
Hint: Always metal loos or donate electrons very easily and the atom which has high electronegativity accepts those electrons donated by the metal atoms. One of the examples of highly electronegativity atoms is oxygen. Oxygen accepts electrons very easily and forms respective oxides as the products.
Complete step by step answer:
The chemicals given in the question are sodium oxide (\[N{{a}_{2}}O\]) and magnesium oxide (MgO).
First we will discuss the formation of \[N{{a}_{2}}O\].
The atomic number of sodium is 11 means sodium contains eleven electrons in its electronic configuration.
The electronic configuration of sodium is as follows
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}\]
Sodium is going to react with oxygen.
Atomic number of oxygen is 8 means oxygen contains eight electrons in its electronic configuration.
The electronic configuration of oxygen is as follows
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}\]
To get octet electronic configuration oxygen accepts two electrons.
To get stable configuration sodium loses one electron and to get stable electronic configuration oxygen accepts two electrons.
So, two sodium atoms loses two electrons and those electrons are going to accept by oxygen to get stable electronic configuration of \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}\].
We can represent the transfer of electrons from sodium to oxygen atoms as follows.
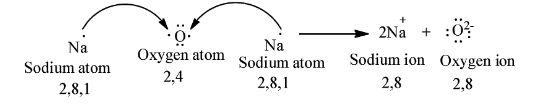
Coming to the formation of MgO.
One mole of magnesium reacts with one oxygen atom and forms Magnesium oxide (MgO).
The atomic number of magnesium is 12 means magnesium contains twelve electrons in its electronic configuration.
The electronic configuration of magnesium is as follows
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}\]
Magnesium is going to react with oxygen.
Atomic number of oxygen is 8 means oxygen contains eight electrons in its electronic configuration.
The electronic configuration of oxygen is as follows
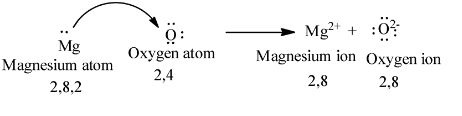
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}\]
To get octet electronic configuration oxygen accepts two electrons.
To get stable configuration magnesium loses two electrons and to get stable electronic configuration oxygen accepts two electrons.
So, one magnesium atom loses two electrons and those electrons are going to accept oxygen to get a stable electronic configuration of \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}\].
We can represent the transfer of electrons from magnesium to oxygen atoms as follows.
Note: In the formation of \[N{{a}_{2}}O\], two moles of sodium react with one mole of oxygen atom and to form MgO, one mole of magnesium reacts with one mole of oxygen atom. When metals react with oxygen then they form respective oxides.
Complete step by step answer:
The chemicals given in the question are sodium oxide (\[N{{a}_{2}}O\]) and magnesium oxide (MgO).
First we will discuss the formation of \[N{{a}_{2}}O\].
The atomic number of sodium is 11 means sodium contains eleven electrons in its electronic configuration.
The electronic configuration of sodium is as follows
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}\]
Sodium is going to react with oxygen.
Atomic number of oxygen is 8 means oxygen contains eight electrons in its electronic configuration.
The electronic configuration of oxygen is as follows
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}\]
To get octet electronic configuration oxygen accepts two electrons.
To get stable configuration sodium loses one electron and to get stable electronic configuration oxygen accepts two electrons.
So, two sodium atoms loses two electrons and those electrons are going to accept by oxygen to get stable electronic configuration of \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}\].
We can represent the transfer of electrons from sodium to oxygen atoms as follows.

Coming to the formation of MgO.
One mole of magnesium reacts with one oxygen atom and forms Magnesium oxide (MgO).
The atomic number of magnesium is 12 means magnesium contains twelve electrons in its electronic configuration.
The electronic configuration of magnesium is as follows
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}\]
Magnesium is going to react with oxygen.
Atomic number of oxygen is 8 means oxygen contains eight electrons in its electronic configuration.
The electronic configuration of oxygen is as follows

\[1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}\]
To get octet electronic configuration oxygen accepts two electrons.
To get stable configuration magnesium loses two electrons and to get stable electronic configuration oxygen accepts two electrons.
So, one magnesium atom loses two electrons and those electrons are going to accept oxygen to get a stable electronic configuration of \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}\].
We can represent the transfer of electrons from magnesium to oxygen atoms as follows.
Note: In the formation of \[N{{a}_{2}}O\], two moles of sodium react with one mole of oxygen atom and to form MgO, one mole of magnesium reacts with one mole of oxygen atom. When metals react with oxygen then they form respective oxides.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




