
Show the electron dot representation of
(A) $ LiCl $
(B) $ CaO $
Answer
535.5k+ views
Hint: We know that the electron dot representation such that the dot shows the valence electrons of the atom. This representation of dots surrounding the atom is known as the electron dot representation of that atom. First of all we have to study the valence and the electronic configuration of that atom and represent that valence electron in the form of dots surrounding that atom.
Complete answer:
(A) $ LiCl $
Here the compound given is $ LiCl $ , for the electron dot configuration or representation we must know about these atoms electronic configuration as:
Electronic configuration of $ Li $ : $ 1{s^2}2{s^1} $
Electronic configuration of $ Cl $ : $ 1{s^2}2{s^2}2{p^6}3{s^2}3{p^5} $
Valence electron of $ Li $ is $ 1 $ and of $ Cl $ is $ 1 $
Therefore, the electron dot representation is given in the following figure as:
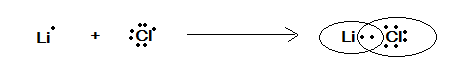
In the above figure we have represented the electron dot representation of $ LiCl $ where these two atoms share a pair of electrons. They both satisfy the valence of each other.
(B) $ CaO $
Here, the compound given is $ CaO $ . So we have to first write the electronic configuration as we have written in the above solution. Thus,
Electronic configuration of $ Ca $ : $ 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2} $
Electronic configuration of $ O $ : $ 1{s^2}2{s^2}2{p^4} $
Valence electron for $ Ca $ is $ 2 $ and of $ O $ is $ 6 $
Therefore, the electron dot representation is given in the following figure as:
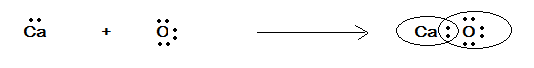
In the above figure we have the electron dot representation of $ CaO $ where these two atoms share a pair of electrons. They both satisfy the valence of each other.
These are the electron dot representation of the given compounds.
Note:
Here, it is necessary to know the configuration of the required element and the valence electrons are represented in the electron dot structure of the compounds as we have done above. We must take care of the valence electrons that means the electrons that are in the outermost shell and here these compounds satisfy each other’s valency by sharing electrons each possess.
Complete answer:
(A) $ LiCl $
Here the compound given is $ LiCl $ , for the electron dot configuration or representation we must know about these atoms electronic configuration as:
Electronic configuration of $ Li $ : $ 1{s^2}2{s^1} $
Electronic configuration of $ Cl $ : $ 1{s^2}2{s^2}2{p^6}3{s^2}3{p^5} $
Valence electron of $ Li $ is $ 1 $ and of $ Cl $ is $ 1 $
Therefore, the electron dot representation is given in the following figure as:

In the above figure we have represented the electron dot representation of $ LiCl $ where these two atoms share a pair of electrons. They both satisfy the valence of each other.
(B) $ CaO $
Here, the compound given is $ CaO $ . So we have to first write the electronic configuration as we have written in the above solution. Thus,
Electronic configuration of $ Ca $ : $ 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2} $
Electronic configuration of $ O $ : $ 1{s^2}2{s^2}2{p^4} $
Valence electron for $ Ca $ is $ 2 $ and of $ O $ is $ 6 $
Therefore, the electron dot representation is given in the following figure as:
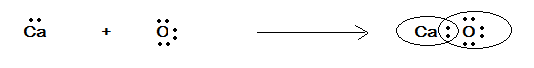
In the above figure we have the electron dot representation of $ CaO $ where these two atoms share a pair of electrons. They both satisfy the valence of each other.
These are the electron dot representation of the given compounds.
Note:
Here, it is necessary to know the configuration of the required element and the valence electrons are represented in the electron dot structure of the compounds as we have done above. We must take care of the valence electrons that means the electrons that are in the outermost shell and here these compounds satisfy each other’s valency by sharing electrons each possess.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

Which out of the following hydrocarbons undergo addition class 11 chemistry CBSE




