
How would you show J.J. Thomson’s model of the atom using small beads and clay?
Answer
536.1k+ views
Hint:J.J. Thomson created a model that inferred a rough idea about the structure of an atom. His model is also known as watermelon, raisin pudding or plum pudding model.
Complete step-by-step answer:J.J. Thomson made a model on the structure of an atom through his assumptions. His assumptions or postulates were:
- The model explained electrical neutrality of an atom.
- the mass of an atom which is positively charged, is uniform throughout the atom.
- the electrons which are negatively charged are embedded inside this positively charged atom.
This model made a representation like a pudding in which raisins are embedded as electrons, or a watermelon with seeds embedded as electrons.
The model can be represented as,
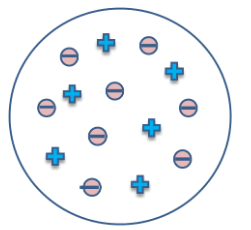
The positive charge can be seen throughout, while the negative electrons are embedded inside.
We can practically make this model using beads and clay. Through clay, a ball can be made in which we can fix small beads as electrons.
Hence, J.J. Thomson’s model can be made from beads and clay.
Additional information: some other scientists also proposed models for the structure of an atom, like Rutherford’s model through his gold foil scattering experiment and Bohr’s model of an atom.
Note:J.J. Thomson’s model was considered as rejected; it was discovered that the positive part in an atom is concentrated in a small amount at the centre of the atom in the nucleus.
Complete step-by-step answer:J.J. Thomson made a model on the structure of an atom through his assumptions. His assumptions or postulates were:
- The model explained electrical neutrality of an atom.
- the mass of an atom which is positively charged, is uniform throughout the atom.
- the electrons which are negatively charged are embedded inside this positively charged atom.
This model made a representation like a pudding in which raisins are embedded as electrons, or a watermelon with seeds embedded as electrons.
The model can be represented as,

The positive charge can be seen throughout, while the negative electrons are embedded inside.
We can practically make this model using beads and clay. Through clay, a ball can be made in which we can fix small beads as electrons.
Hence, J.J. Thomson’s model can be made from beads and clay.
Additional information: some other scientists also proposed models for the structure of an atom, like Rutherford’s model through his gold foil scattering experiment and Bohr’s model of an atom.
Note:J.J. Thomson’s model was considered as rejected; it was discovered that the positive part in an atom is concentrated in a small amount at the centre of the atom in the nucleus.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




