
$S{{F}_{4}}$ has ____________ shape.
(A) T-Shape
(B) Bend
(C) Octahedral
(D) see-saw
Answer
590.4k+ views
Hint: Use VSEPR Theory postulates such as pair of electrons in valence shell repel one another since their cloud is negatively charged and if there is lone pair that means more repulsion since $S{{F}_{4}}$ also have one lone pair.
Complete answer:
As says the VEPER Theory
A lone pair of electrons takes up more space round the central atom than a bond pair, since the lone pair is attracted to one nucleus while the bond pair is shared by two nuclei. Thus, the presence of lone pairs causes changes such as:
\[bond\text{ }pair\left( bp \right)\text{ }\text{ }bond\text{ }pair\left( bp \right)~<\text{ }lone\text{ }pair\left( lp \right)\text{ }\text{ }bond\text{ }pair\left( bp \right)~~~<\text{ }Lone\text{ }pair\left( lp \right)~\text{ }lone\text{ }pair\left( lp \right)\]
As we know $S{{F}_{4}}$ has 4-bond pairs and 1-lone pair so it’s assumed geometry should be Trigonal bi-pyramidal but due to its lone pair its shape more like a see-saw because lone pair-bond pair repulsion is much more than the bond pair-bond pair repulsion so the angle is reduced to ${{102}^{\circ }}$ from ${{120}^{\circ }}$.
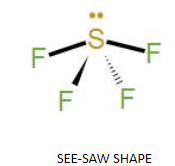
Besides this we can also see that in (a) the lone pair is present at axial position so there are three lone pair-bond repulsions at ${{90}^{\circ }}$. In (b) the lone pair is in equatorial position and there are two lone pairs –bond pair repulsion. Hence (b) is more stable which is also called as a distorted tetrahedron, a folded square and a see-saw.

Hence, among the options if we check option (D) i.e. See Saw is the correct answer for this question.
Note: VSEPR theory has certain postulates which one must follow to deduce the geometrical structure of a compound.
Here $S{{F}_{4}}$ has one lone pair of electrons. The presence of a lone pair changes the actual angle. Always remember that the more stable structure is preferable, so even if we have different possibilities only the most stable one among them is considered.
Complete answer:
As says the VEPER Theory
A lone pair of electrons takes up more space round the central atom than a bond pair, since the lone pair is attracted to one nucleus while the bond pair is shared by two nuclei. Thus, the presence of lone pairs causes changes such as:
\[bond\text{ }pair\left( bp \right)\text{ }\text{ }bond\text{ }pair\left( bp \right)~<\text{ }lone\text{ }pair\left( lp \right)\text{ }\text{ }bond\text{ }pair\left( bp \right)~~~<\text{ }Lone\text{ }pair\left( lp \right)~\text{ }lone\text{ }pair\left( lp \right)\]
As we know $S{{F}_{4}}$ has 4-bond pairs and 1-lone pair so it’s assumed geometry should be Trigonal bi-pyramidal but due to its lone pair its shape more like a see-saw because lone pair-bond pair repulsion is much more than the bond pair-bond pair repulsion so the angle is reduced to ${{102}^{\circ }}$ from ${{120}^{\circ }}$.

Besides this we can also see that in (a) the lone pair is present at axial position so there are three lone pair-bond repulsions at ${{90}^{\circ }}$. In (b) the lone pair is in equatorial position and there are two lone pairs –bond pair repulsion. Hence (b) is more stable which is also called as a distorted tetrahedron, a folded square and a see-saw.

Hence, among the options if we check option (D) i.e. See Saw is the correct answer for this question.
Note: VSEPR theory has certain postulates which one must follow to deduce the geometrical structure of a compound.
Here $S{{F}_{4}}$ has one lone pair of electrons. The presence of a lone pair changes the actual angle. Always remember that the more stable structure is preferable, so even if we have different possibilities only the most stable one among them is considered.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




