
Select the correct statements about diborane :
(a) $ {B_2}{H_6} $ has three centred bound
(b) each boron atom lies in $ s{p^3} $ hybrid state
(c) $ {H_b} $ . . . . . B . . . . . . $ {H_b} $ bond angle is $ {122^o} $
(d) All hydrogens in $ {B_2}{H_6} $ lie the same.
Answer
512.1k+ views
Hint :The chemical compound diborane, sometimes known as diborane, has the formula $ {B_2}{H_6} $ and is made up of boron and hydrogen. It's a colourless, pyrophoric gas with an odiferous sweet odour. Diborane is a common boron compound with a wide range of uses. Its electronic structure has gotten a lot of interest. It has a number of derivatives that are valuable reagents.
Complete Step By Step Answer:
The Diborane molecule is made up of four hydrogen atoms and two boron atoms that are all in the same plane. Two splitting hydrogen atoms are thought to exist between these planes. The boron atom possesses four hybrid orbitals and is known to be $ s{p^3} $ hybridised. Three of the four hybrid orbitals have one electron apiece, with the fourth orbital being an empty orbital. The two hybrid orbital electrons in each boron atom make two bonds with the 1s hydrogen atoms. The two boron atoms remaining with each unpaired electron orbital and empty orbital create two bridgings (B–H–B) bonds with the two 1s hydrogen atoms, which is also known as the banana bond.
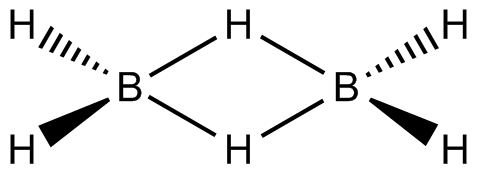
The links between boron and the terminal hydrogen atoms are described by molecular orbital theory as standard 2-center 2-electron covalent bonds. The connection between the boron atoms and the bridging hydrogen atoms, on the other hand, is not the same as in hydrocarbon molecules. Each boron bonds to the terminal hydrogen atoms with two electrons and has one valence electron left over for further bonding. Each of the bridging hydrogen atoms contributes one electron. Four electrons make two 3-center 2-electron bonds that hold the $ {B_2}{H_2} $ ring together. A "banana bond" is a term used to describe this form of relationship.
Hence options A B C are correct.
Note :
Because the 12 valence electrons can only form 6 typical 2-centre 2-electron bonds, which are inadequate to bind all 8 atoms, diborane has traditionally been classified as electron-deficient. The more accurate description utilising 3-centre bonds, on the other hand, reveals that diborane is really electron-precise, with just enough valence electrons to cover the 6 bonding molecular orbitals.
Complete Step By Step Answer:
The Diborane molecule is made up of four hydrogen atoms and two boron atoms that are all in the same plane. Two splitting hydrogen atoms are thought to exist between these planes. The boron atom possesses four hybrid orbitals and is known to be $ s{p^3} $ hybridised. Three of the four hybrid orbitals have one electron apiece, with the fourth orbital being an empty orbital. The two hybrid orbital electrons in each boron atom make two bonds with the 1s hydrogen atoms. The two boron atoms remaining with each unpaired electron orbital and empty orbital create two bridgings (B–H–B) bonds with the two 1s hydrogen atoms, which is also known as the banana bond.

The links between boron and the terminal hydrogen atoms are described by molecular orbital theory as standard 2-center 2-electron covalent bonds. The connection between the boron atoms and the bridging hydrogen atoms, on the other hand, is not the same as in hydrocarbon molecules. Each boron bonds to the terminal hydrogen atoms with two electrons and has one valence electron left over for further bonding. Each of the bridging hydrogen atoms contributes one electron. Four electrons make two 3-center 2-electron bonds that hold the $ {B_2}{H_2} $ ring together. A "banana bond" is a term used to describe this form of relationship.
Hence options A B C are correct.
Note :
Because the 12 valence electrons can only form 6 typical 2-centre 2-electron bonds, which are inadequate to bind all 8 atoms, diborane has traditionally been classified as electron-deficient. The more accurate description utilising 3-centre bonds, on the other hand, reveals that diborane is really electron-precise, with just enough valence electrons to cover the 6 bonding molecular orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




