
Select the correct order of boiling point:
A. Diethyl ether>n-butyl alcohol>n-butyraldehyde>n-pentane
B. n-butyl alcohol>n-butyraldehyde>n-pentane>diethyl ether
C. n-pentane>n-butyraldehyde>n-butyl alcohol>diethyl ether
D. n-butyl alcohol>n-butyraldehyde>diethyl ether>n-pentane
Answer
588k+ views
Hint: Boiling point is defined as the temperature at which a liquid starts to boil. In the case of organic compounds, boiling point is dependent upon polarity, molecular mass and intermolecular force of attraction.
Complete step by step answer:
In case of the above question we have alcohol, aldehyde, ether and hydrocarbon i.s. alkane. Let’s discuss each case one by one.
In case of alcohol there is a hydrogen atom which is attached to a strongly electronegative atom, i.e. oxygen atom. Hence, intermolecular hydrogen bonding is observed. So, they have higher boiling point as alcohol exists as an associated molecule. The molecular diagram of n-butyl alcohol is given below:
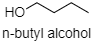
In case of aldehyde due to presence of polar carbonyl group, weak intermolecular association is observed due to dipole-dipole interaction between the opposite ends of carbonyl dipoles. The molecular diagram of n-butyraldehyde is given below:

Since in case of alcohol there is more extensive hydrogen bonding hence it has greater boiling point than aldehyde.
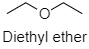
Now in case of ether there is no hydrogen bonding present. Hence there is no molecular association. So ether has a low boiling point as compared to alcohol. The molecular diagram for Diethyl ether is shown below:
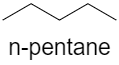
There is no hydrogen bonding in case of n-pentane, as shown below, so it has low boiling point but the molecular mass of n-pentane is more than diethyl ether. Hence, it has more boiling point as compared to diethyl ether.
The correct answer is option (b).
Note: Hydrogen bonding is defined as the bond between hydrogen and most electronegative atoms F, O and N. Dipole-dipole interaction is weaker than hydrogen bonding. So, the boiling point of organic compounds having hydrogen bonding is larger than compounds having dipole-dipole interaction.
Complete step by step answer:
In case of the above question we have alcohol, aldehyde, ether and hydrocarbon i.s. alkane. Let’s discuss each case one by one.
In case of alcohol there is a hydrogen atom which is attached to a strongly electronegative atom, i.e. oxygen atom. Hence, intermolecular hydrogen bonding is observed. So, they have higher boiling point as alcohol exists as an associated molecule. The molecular diagram of n-butyl alcohol is given below:
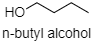
In case of aldehyde due to presence of polar carbonyl group, weak intermolecular association is observed due to dipole-dipole interaction between the opposite ends of carbonyl dipoles. The molecular diagram of n-butyraldehyde is given below:

Since in case of alcohol there is more extensive hydrogen bonding hence it has greater boiling point than aldehyde.
Now in case of ether there is no hydrogen bonding present. Hence there is no molecular association. So ether has a low boiling point as compared to alcohol. The molecular diagram for Diethyl ether is shown below:

There is no hydrogen bonding in case of n-pentane, as shown below, so it has low boiling point but the molecular mass of n-pentane is more than diethyl ether. Hence, it has more boiling point as compared to diethyl ether.

The correct answer is option (b).
Note: Hydrogen bonding is defined as the bond between hydrogen and most electronegative atoms F, O and N. Dipole-dipole interaction is weaker than hydrogen bonding. So, the boiling point of organic compounds having hydrogen bonding is larger than compounds having dipole-dipole interaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




