
Select the correct option(s).
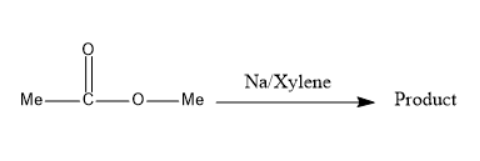
(A) Product is optically inactive
(B) Product is optically active
(C) In the given reaction free radical is formed as intermediate
(D) Product shows tautomerism
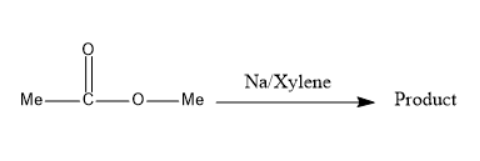
Answer
487.5k+ views
Hint: Esters are the chemical compounds consisting of $ RCOO{R'} $ , when two molecules of esters are reacted in presence of sodium and xylene, the product formed consists of a ketone and alcohol functional group which can undergo tautomerism. The product formed via the formation of a free radical intermediate.
Complete answer:
Chemical compounds are classified into functional groups based on the groups present in it. Esters are the chemical compounds consisting of $ RCOO{R'} $ , where $ R $ and $ {R'} $ are sometimes same alkyl groups and sometimes different alkyl groups.
Two molecules of esters in presence of sodium and xylene form a product known as $ 3 $ -hydroxy butane $ 2 $ -one. Further this product undergoes proton shift which can be known as tautomerism and forms a product named as $ but - 2 - ene - 2,3 - diol $ .
The compound formed is a racemic mixture which means equimolar of enantiomers. Thus, the product is optically inactive. This reaction can be passed through the formation of a free radical intermediate.
Thus, option A, option C, and option D are the correct options.
Note:
Aprotic solvents like xylene, benzene, and toluene were not used in the reaction. It leads to the formation of other products rather than this acyloin condensation. When protic solvents like ethanol, water are used it proceeds a chemical reaction known as Bouveault-Blanc reduction in which an ester is reduced to an alcohol.
Complete answer:
Chemical compounds are classified into functional groups based on the groups present in it. Esters are the chemical compounds consisting of $ RCOO{R'} $ , where $ R $ and $ {R'} $ are sometimes same alkyl groups and sometimes different alkyl groups.
Two molecules of esters in presence of sodium and xylene form a product known as $ 3 $ -hydroxy butane $ 2 $ -one. Further this product undergoes proton shift which can be known as tautomerism and forms a product named as $ but - 2 - ene - 2,3 - diol $ .
The compound formed is a racemic mixture which means equimolar of enantiomers. Thus, the product is optically inactive. This reaction can be passed through the formation of a free radical intermediate.
Thus, option A, option C, and option D are the correct options.
Note:
Aprotic solvents like xylene, benzene, and toluene were not used in the reaction. It leads to the formation of other products rather than this acyloin condensation. When protic solvents like ethanol, water are used it proceeds a chemical reaction known as Bouveault-Blanc reduction in which an ester is reduced to an alcohol.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




