
Select the correct option according to Hund’s rule and Pauli’s exclusion principle.
I.
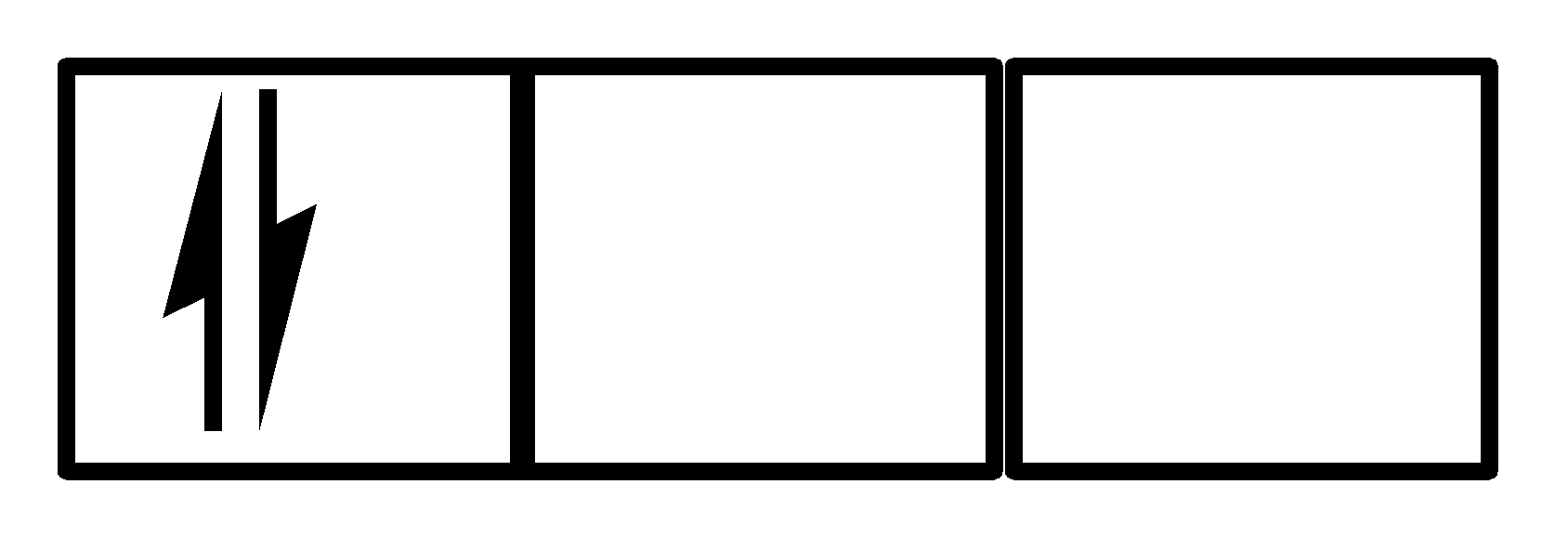
II.
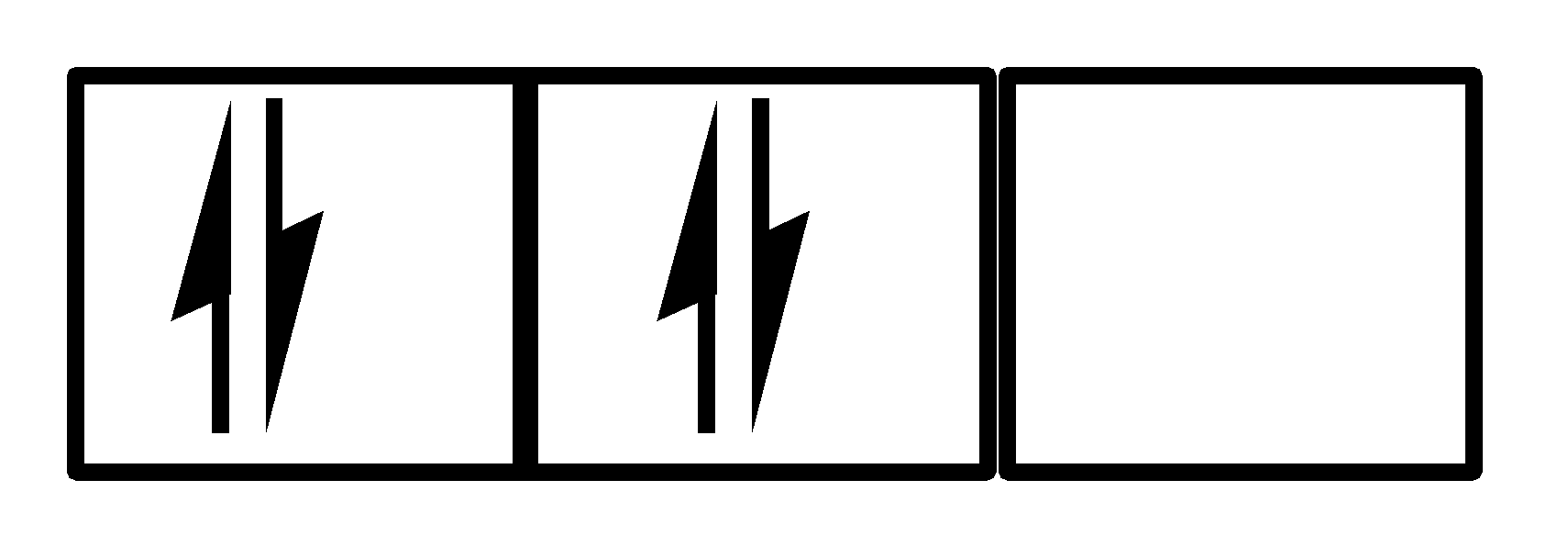
III.
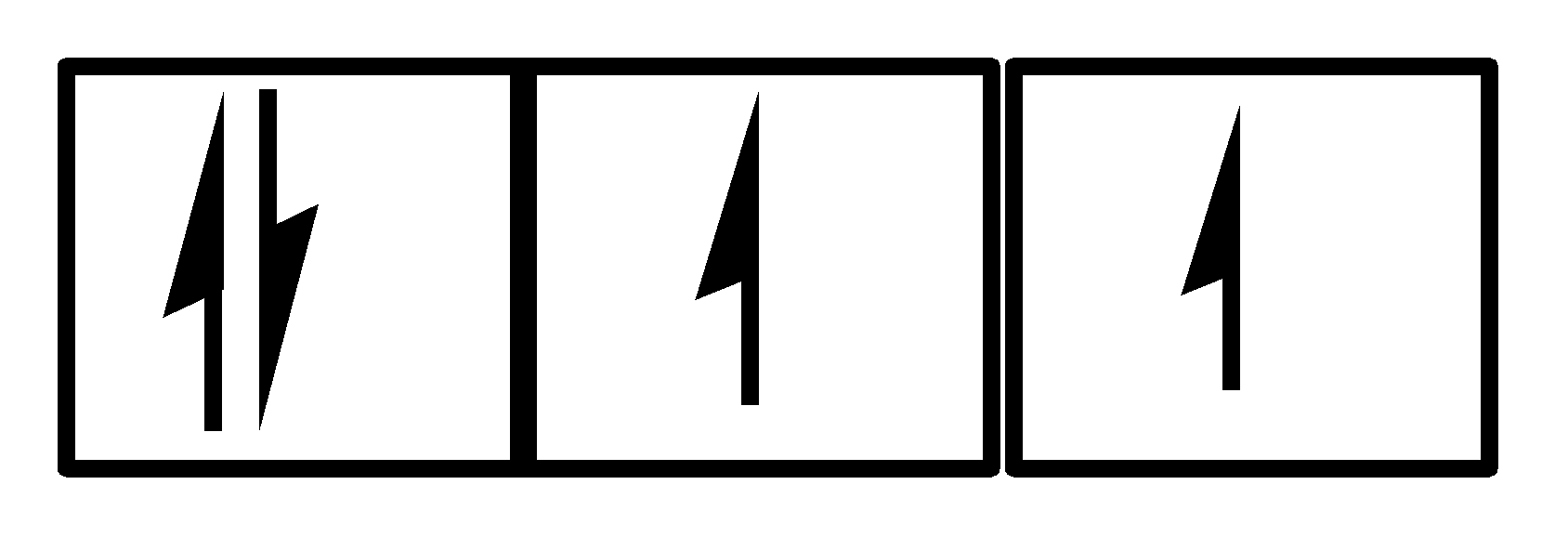
IV.
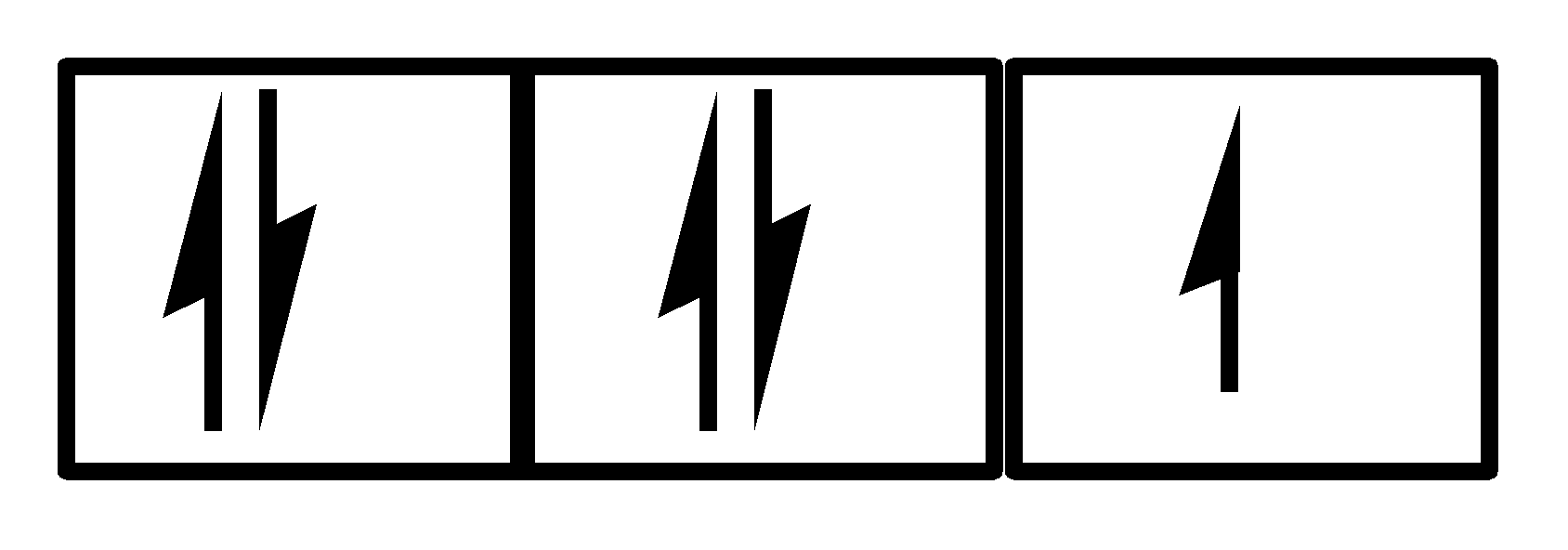
A.Both I and II
B.Both II and III
C.Both I and III
D.Both III and IV




Answer
587.1k+ views
Hint: We must know that the Hund’s Rule states that every orbital in a subshell is first singly occupied by the electron before being occupied by the second electron. Pauli’s exclusion principle, no two electrons have the same set of quantum numbers.
Complete step by step answer:
Let’s start with discussing the Hund’s rule and Pauli’s exclusion principle, Hund’s Rule states that every orbital in a subshell is first singly occupied by the electron before being occupied by the second electron. In easy words, only one electron enters each orbital and after each orbital is having a single electron each, only then the second electron enters the orbitals. According to Pauli’s exclusion principle, no two electrons have the same set of quantum numbers. Means, if there are two electrons in the same subshell; same orbital then their spin will be different.
So, considering both the rules, let’s solve the question.
In I
Both the electrons are present in the 1st orbital in opposite spins, so Pauli’s exclusion principle is valid in this case but Hund’s rule has been violated, which means I. is not a correct option.
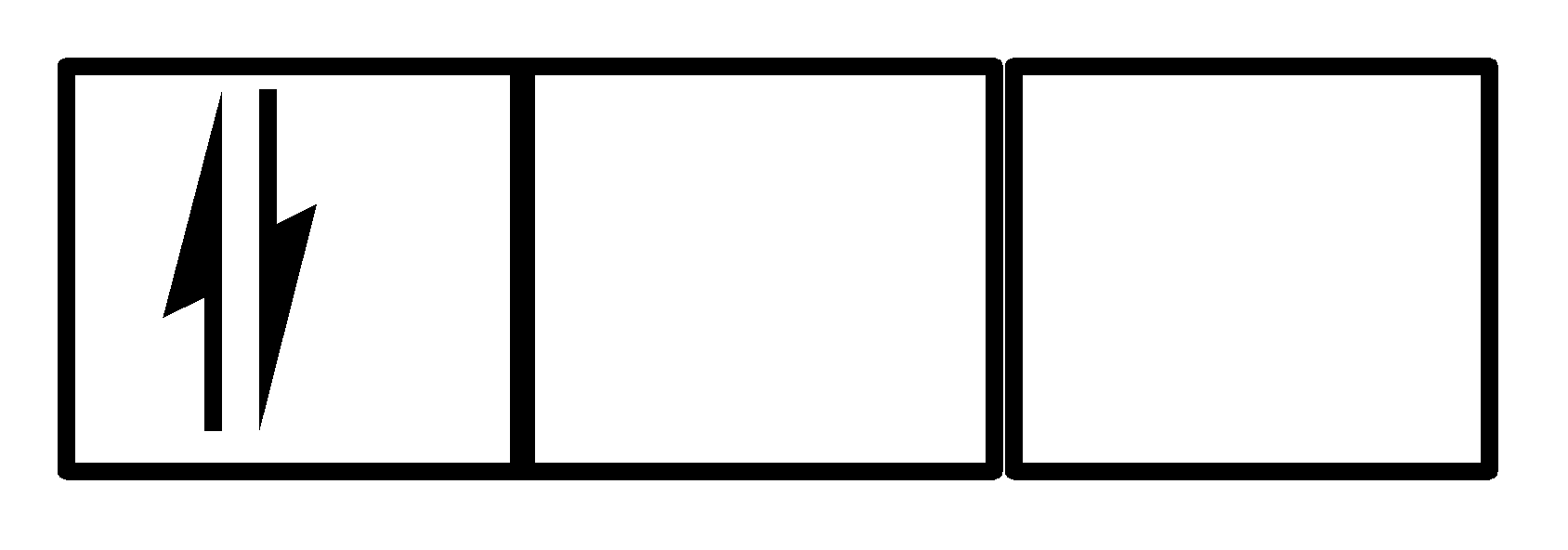
In II
There are 4 electrons and they are present in the first two orbitals, Pauli’s exclusion principle is being followed but hund’s rule has been violated, which means II. This is also not a correct option.
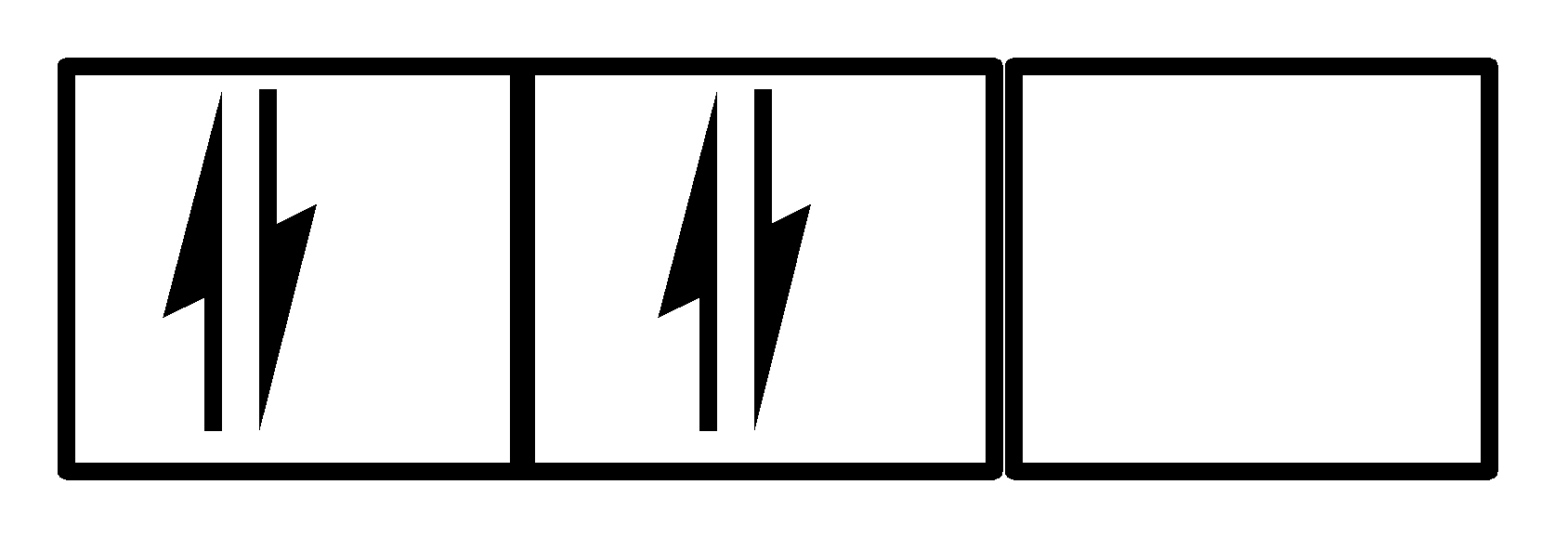
In III
There are also 4 electrons and all three orbitals are being used along with 2 electrons in the 1st orbital and one each in the 2nd and 3rd orbital. Clearly, both the Hund’s rule and Pauli’s exclusion principle is being followed. So, III is a correct option.
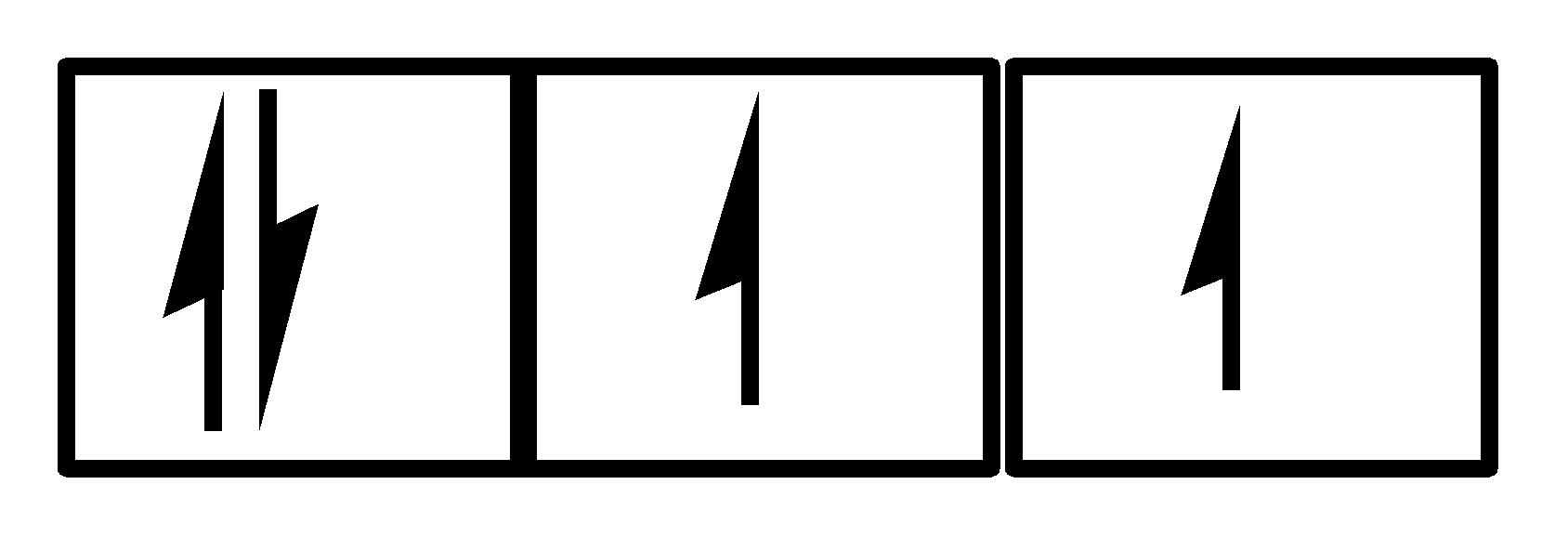
In IV
There are 5 electrons and all 3 orbitals are being used. Clearly, Hund’s rule and Pauli’s exclusion principle is being followed. So, option IV is also correct.
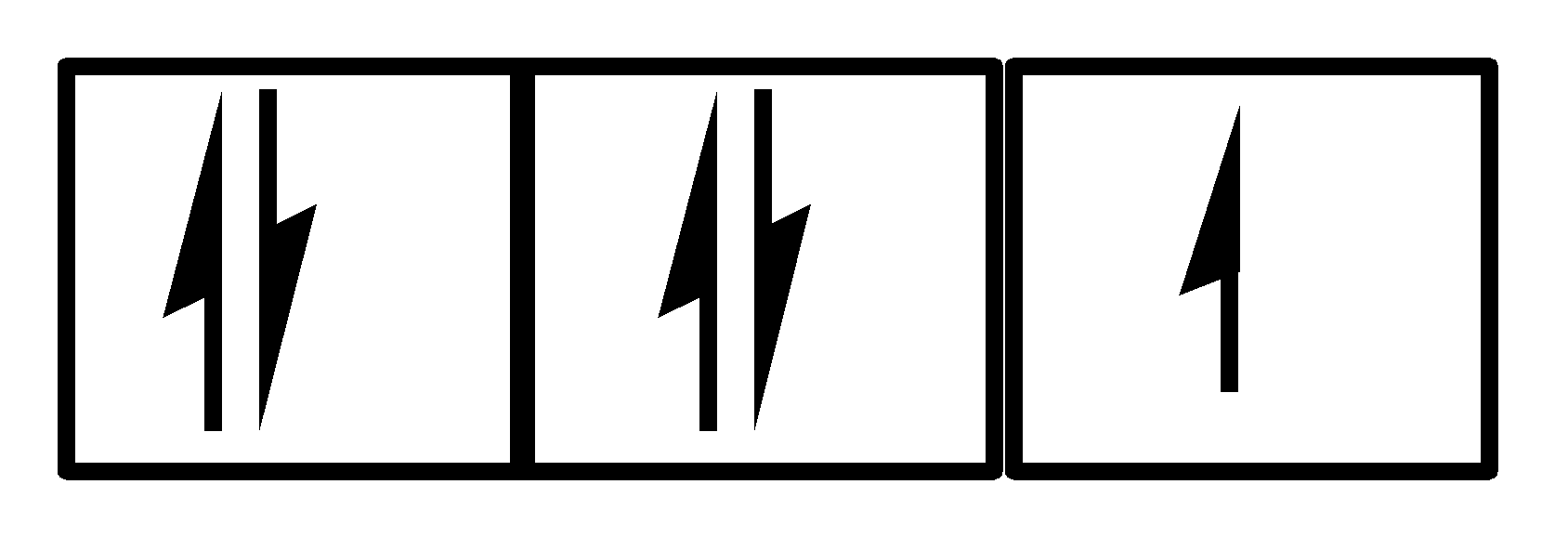
Hence, the answer to this question is option D. both III and IV.
Note:
We can use both the Hund’s Rule and Pauli’s exclusion principle to understand how the electrons are placed in orbitals and how to place them inside the orbitals. They are also important to understand that each electron in the atom has their own identity, which is known as Pauli’s exclusion principle.
Complete step by step answer:
Let’s start with discussing the Hund’s rule and Pauli’s exclusion principle, Hund’s Rule states that every orbital in a subshell is first singly occupied by the electron before being occupied by the second electron. In easy words, only one electron enters each orbital and after each orbital is having a single electron each, only then the second electron enters the orbitals. According to Pauli’s exclusion principle, no two electrons have the same set of quantum numbers. Means, if there are two electrons in the same subshell; same orbital then their spin will be different.
So, considering both the rules, let’s solve the question.
In I
Both the electrons are present in the 1st orbital in opposite spins, so Pauli’s exclusion principle is valid in this case but Hund’s rule has been violated, which means I. is not a correct option.

In II
There are 4 electrons and they are present in the first two orbitals, Pauli’s exclusion principle is being followed but hund’s rule has been violated, which means II. This is also not a correct option.

In III
There are also 4 electrons and all three orbitals are being used along with 2 electrons in the 1st orbital and one each in the 2nd and 3rd orbital. Clearly, both the Hund’s rule and Pauli’s exclusion principle is being followed. So, III is a correct option.

In IV
There are 5 electrons and all 3 orbitals are being used. Clearly, Hund’s rule and Pauli’s exclusion principle is being followed. So, option IV is also correct.

Hence, the answer to this question is option D. both III and IV.
Note:
We can use both the Hund’s Rule and Pauli’s exclusion principle to understand how the electrons are placed in orbitals and how to place them inside the orbitals. They are also important to understand that each electron in the atom has their own identity, which is known as Pauli’s exclusion principle.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




