
Select correct IUPAC naming of given compound.
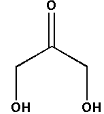
(A) Propanone-1,3-diol
(B) 1,3-dihydroxy propanal
(C) 1,3-dihydroxypropanone
(D) Propanediol

Answer
567k+ views
Hint:The method of naming organic compounds is known as the IUPAC nomenclature. In the given structure, two hydroxyl i.e. alcoholic groups are attached as substituents. Also, there is one carbonyl i.e. ketonic group present.
Complete step-by-step solution:The given structure with all of its atoms is as follows:
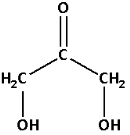
Select the longest continuous chain of carbon atoms in the structure of the molecule.
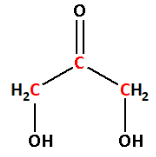
Number the carbon atoms in the selected carbon chain from the end which is nearest to the hydroxyl substituents. But the hydroxyl substituents are attached to both the ends. Thus, we can name the carbon atoms from any side.
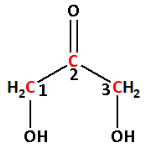
The longest chain contains three carbon atoms. Thus, the parent alkane is propane. And one ketonic group is attached to carbon number-2.Thus, propanone.
The two hydroxyl groups are attached to carbon number -1 and carbon number- 3. Thus, 1,3-dihydroxy.
Thus, the IUPAC name is 1,3-dihydroxypropanone.
Thus, the correct option is (C) 1,3-dihydroxypropanone.
Thus, the correct option is (C) 1,3-dihydroxypropane.
Note:The rules for writing IUPAC name of compounds are as follows:
1. Select the longest continuous chain of carbon atoms in the structure of the molecule.
2. Number the carbon atoms in the selected carbon chain from the end which is nearest to the substituents attached.
3. Count the number of carbon atoms in the chain. This is the parent alkane.
4. Write the number indicating the position of the substituents.
5. Assign a number to each substituent according to the carbon atom it is attached. If there are two substituents on the same carbon, assign the same number to them.
Complete step-by-step solution:The given structure with all of its atoms is as follows:

Select the longest continuous chain of carbon atoms in the structure of the molecule.

Number the carbon atoms in the selected carbon chain from the end which is nearest to the hydroxyl substituents. But the hydroxyl substituents are attached to both the ends. Thus, we can name the carbon atoms from any side.

The longest chain contains three carbon atoms. Thus, the parent alkane is propane. And one ketonic group is attached to carbon number-2.Thus, propanone.
The two hydroxyl groups are attached to carbon number -1 and carbon number- 3. Thus, 1,3-dihydroxy.
Thus, the IUPAC name is 1,3-dihydroxypropanone.
Thus, the correct option is (C) 1,3-dihydroxypropanone.
Thus, the correct option is (C) 1,3-dihydroxypropane.
Note:The rules for writing IUPAC name of compounds are as follows:
1. Select the longest continuous chain of carbon atoms in the structure of the molecule.
2. Number the carbon atoms in the selected carbon chain from the end which is nearest to the substituents attached.
3. Count the number of carbon atoms in the chain. This is the parent alkane.
4. Write the number indicating the position of the substituents.
5. Assign a number to each substituent according to the carbon atom it is attached. If there are two substituents on the same carbon, assign the same number to them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




