
Secondary nitro compounds when react with $ HN{O_2} $ forms crystalline solids which on treatment with $ NaOH $ gives
A. Red solution
B. Blue solution
C. White precipitate
D. Yellow colouration
Answer
513.9k+ views
Hint :Nitroalkanes are the organic compounds which are colourless pleasant-smelling liquids and are formed on reaction of a haloalkane with silver nitrate. The general chemical formula of a nitroalkane is $ {C_n}{H_{2n + 1}}N{O_2} $ . The lower nitroalkanes (like nitromethane) are used for internal combustion engines as high-performance fuels, as polar solvents and as chemical intermediates.
Complete Step By Step Answer:
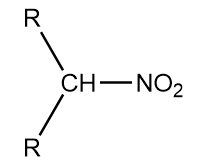
Secondary nitro compounds: When two alkyl groups are attached to the carbon atom that is adjacent to the nitro group, then the compound is termed as secondary nitro compound or secondary nitroalkane. Structurally, it is expressed as follows:
When secondary nitro compounds react with nitrous acid, then the formation of pseudo nitriles takes place along with the removal of water. The reaction takes place as follows:
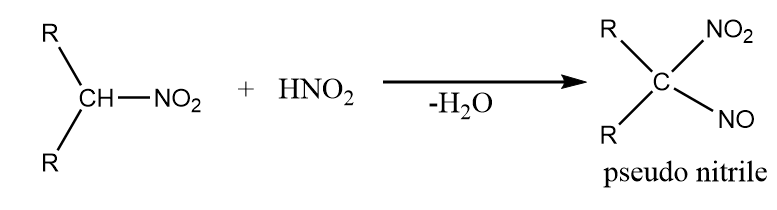
On further reaction of pseudo nitriles with $ NaOH $ , a blue coloured solution is obtained as a product.
Hence, option (B) is the correct answer.
Additional Information:
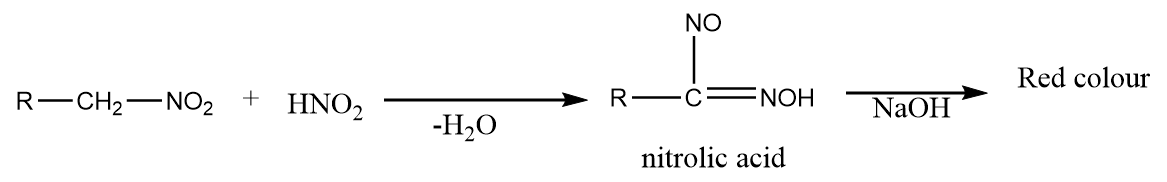
When primary nitro compounds react with nitrous acid, then they form nitrolic acid which on further treatment with sodium hydroxide, yields a deep red colour solution. The reaction is as follows:
Note :
It is important to note that the tertiary nitro compounds do not give any reaction with nitrous acid because there is no hydrogen present on the carbon atom and therefore, removal of water is not possible in this case. These reactions are a very important lab reaction as these are used to distinguish between primary, secondary and tertiary alcohols.
Complete Step By Step Answer:
Secondary nitro compounds: When two alkyl groups are attached to the carbon atom that is adjacent to the nitro group, then the compound is termed as secondary nitro compound or secondary nitroalkane. Structurally, it is expressed as follows:

When secondary nitro compounds react with nitrous acid, then the formation of pseudo nitriles takes place along with the removal of water. The reaction takes place as follows:

On further reaction of pseudo nitriles with $ NaOH $ , a blue coloured solution is obtained as a product.
Hence, option (B) is the correct answer.
Additional Information:
When primary nitro compounds react with nitrous acid, then they form nitrolic acid which on further treatment with sodium hydroxide, yields a deep red colour solution. The reaction is as follows:

Note :
It is important to note that the tertiary nitro compounds do not give any reaction with nitrous acid because there is no hydrogen present on the carbon atom and therefore, removal of water is not possible in this case. These reactions are a very important lab reaction as these are used to distinguish between primary, secondary and tertiary alcohols.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




