
Schottky defects lower the density of related solids. Why?
Answer
533.1k+ views
Hint: Any solid crystal of a compound is not perfect, means they consist of defects when crystallization occurs at a fast or moderate rate. Defects are the irregularities that arise in the arrangement of constituent particles in a lattice.
Complete answer:
Defects are the irregular arrangement of particles in a crystal lattice. They may arise due to defective crystallization, which makes up the molecule. As a result, many vacancies or voids or even impurities may take the places in the crystal lattice of a substance.
Defects can be of various types, one such is the point defect where the irregularity in arrangement is at one ideal point in the crystal. These defects give rise to stoichiometric defects that disturb the stoichiometry of the crystal.
Schottky defect is a type of stoichiometric defect. This defect occurs in ionic solids. It occurs in compounds where the size of anion and cation is almost similar. In this type of defect equal numbers of anions and cations are missing from the crystal lattice. As depicted in the following diagram,
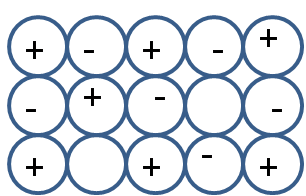
The equal number of missing anion and cation decreases the overall density. As density is mass upon volume, the missing ions decrease the mass, hence the density in schottky defects also decreases.
Note:
Schottky defects can be seen in the compounds like alkali -metal halides, where the cation and anion are of almost similar size. Some examples of compounds with this type of defects are NaCl, KCl, CsCl, and AgBr. The volume in these types of defects remains constant.
Complete answer:
Defects are the irregular arrangement of particles in a crystal lattice. They may arise due to defective crystallization, which makes up the molecule. As a result, many vacancies or voids or even impurities may take the places in the crystal lattice of a substance.
Defects can be of various types, one such is the point defect where the irregularity in arrangement is at one ideal point in the crystal. These defects give rise to stoichiometric defects that disturb the stoichiometry of the crystal.
Schottky defect is a type of stoichiometric defect. This defect occurs in ionic solids. It occurs in compounds where the size of anion and cation is almost similar. In this type of defect equal numbers of anions and cations are missing from the crystal lattice. As depicted in the following diagram,

The equal number of missing anion and cation decreases the overall density. As density is mass upon volume, the missing ions decrease the mass, hence the density in schottky defects also decreases.
Note:
Schottky defects can be seen in the compounds like alkali -metal halides, where the cation and anion are of almost similar size. Some examples of compounds with this type of defects are NaCl, KCl, CsCl, and AgBr. The volume in these types of defects remains constant.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




