
What is Saytzeff (Zaitser) rule? Explain elimination reaction in 2-bromopentane.
Answer
569.4k+ views
Hint:You can use the Saytzeff (Zaitser) rule to determine the major product in the dehydrohalogenation reaction. You can carry out the dehydrohalogenation reaction when you heat an alkyl halide with alcoholic potassium hydroxide.
Complete answer:
When an alkyl halide is heated with aqueous potassium hydroxide solution, it undergoes substitution reaction to form an alcohol.
When an alkyl halide is heated with alcoholic potassium hydroxide solution, it undergoes elimination reaction to form an alkene.
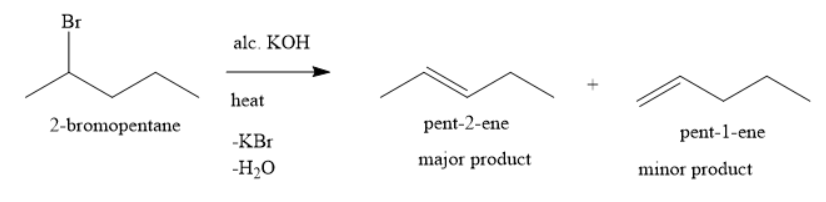
When you heat 2-bromopentane with alcoholic potassium hydroxide, you get a mixture of pent-1-ene and pent-2-ene. In this mixture of alkenes, one is the major product and the other is a minor product. You can identify the major product by applying the Saytzeff (Zaitser) rule. According to this rule, when you carry out the dehydrohalogenation reaction, the major product will be the more substituted alkene and the minor product will be less substituted carbon atom. Here more substituted alkenes means that the alkene, in which more number of alkyl groups are attached to the doubly bonded carbon atom and the less substituted alkenes means that the alkene, in which less number of alkyl groups are attached to the doubly bonded carbon atom.
Among pent-2-ene and pent-1-ene, the more substituted alkene is pent-2-ene. In pent-2-ene, each doubly bonded carbon atom has one alkyl substituent each. In pent-1-ene, one doubly bonded carbon atom has one alkyl substituent whereas other doubly bonded carbon atoms have zero alkyl substituent.
Pent-2-ene being the more substituted alkene is the major product whereas pent-1-ene being the less substituted alkene is the minor product.
Note:
If you use a bulky base, or if you have a bulky leaving group, then the elimination will take place according to Hofmann’s rule. Less substituted alkene will be the major product and more substituted alkene will be the minor product.
Complete answer:
When an alkyl halide is heated with aqueous potassium hydroxide solution, it undergoes substitution reaction to form an alcohol.
When an alkyl halide is heated with alcoholic potassium hydroxide solution, it undergoes elimination reaction to form an alkene.
When you heat 2-bromopentane with alcoholic potassium hydroxide, you get a mixture of pent-1-ene and pent-2-ene. In this mixture of alkenes, one is the major product and the other is a minor product. You can identify the major product by applying the Saytzeff (Zaitser) rule. According to this rule, when you carry out the dehydrohalogenation reaction, the major product will be the more substituted alkene and the minor product will be less substituted carbon atom. Here more substituted alkenes means that the alkene, in which more number of alkyl groups are attached to the doubly bonded carbon atom and the less substituted alkenes means that the alkene, in which less number of alkyl groups are attached to the doubly bonded carbon atom.
Among pent-2-ene and pent-1-ene, the more substituted alkene is pent-2-ene. In pent-2-ene, each doubly bonded carbon atom has one alkyl substituent each. In pent-1-ene, one doubly bonded carbon atom has one alkyl substituent whereas other doubly bonded carbon atoms have zero alkyl substituent.
Pent-2-ene being the more substituted alkene is the major product whereas pent-1-ene being the less substituted alkene is the minor product.

Note:
If you use a bulky base, or if you have a bulky leaving group, then the elimination will take place according to Hofmann’s rule. Less substituted alkene will be the major product and more substituted alkene will be the minor product.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




