
How many same atoms of $PC{l_3}{F_2}$ lie in the same plane?
Answer
586.2k+ views
Hint: We can find the number of atoms lying in the same plane by drawing its geometry with the help of hybridization, i.e. by finding the number of orbitals which the atoms lie. When the atoms lie in the same plane then it is said to be planar.
Complete step by step answer:
Let us start by finding the hybridization of the above structure. The formula used for hybridization is
$X = \dfrac{1}{2}[V + M - C + A]$
Where, V= number of valence electrons of the central atom.
M= number of monovalent atom
C= number of positive charge
A= number of negative charges.
In the case of $PC{l_3}{F_2}$, The central metal atom is $P$ since it belongs to the nitrogen family so the number of valence electrons in $P$ is 5. There are 3 monovalent chlorine atoms and 2 monovalent fluorine atoms. Hence the total monovalent atoms are 5 and there is no overall positive of negative charge on the compound. Hence the hybridization will be $s{p^3}d$ and it can be calculated as below:
$
X = \dfrac{1}{2}[5 + 5] \\
\Rightarrow X = \dfrac{1}{2} \times 10 \\
\Rightarrow X = 5 \\
$
Hence, its geometry will be trigonal bipyramidal and the structure is shown below:
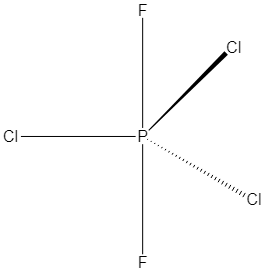
Here three chlorine atoms are in equatorial positions and two fluorine atoms are in axial position. So three same atoms of chlorine of $PC{l_3}{F_2}$ lie in the same plane.
Note:
The bond length of the axial bond is greater than the equatorial bond because the axial bond pair is repelled by three equatorial bond pairs whereas the equatorial bond pair is repelled by the axial bond pair. But the repulsion experienced by the axial bond pair is more.
Complete step by step answer:
Let us start by finding the hybridization of the above structure. The formula used for hybridization is
$X = \dfrac{1}{2}[V + M - C + A]$
Where, V= number of valence electrons of the central atom.
M= number of monovalent atom
C= number of positive charge
A= number of negative charges.
In the case of $PC{l_3}{F_2}$, The central metal atom is $P$ since it belongs to the nitrogen family so the number of valence electrons in $P$ is 5. There are 3 monovalent chlorine atoms and 2 monovalent fluorine atoms. Hence the total monovalent atoms are 5 and there is no overall positive of negative charge on the compound. Hence the hybridization will be $s{p^3}d$ and it can be calculated as below:
$
X = \dfrac{1}{2}[5 + 5] \\
\Rightarrow X = \dfrac{1}{2} \times 10 \\
\Rightarrow X = 5 \\
$
Hence, its geometry will be trigonal bipyramidal and the structure is shown below:

Here three chlorine atoms are in equatorial positions and two fluorine atoms are in axial position. So three same atoms of chlorine of $PC{l_3}{F_2}$ lie in the same plane.
Note:
The bond length of the axial bond is greater than the equatorial bond because the axial bond pair is repelled by three equatorial bond pairs whereas the equatorial bond pair is repelled by the axial bond pair. But the repulsion experienced by the axial bond pair is more.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




