
How many rings are present in ${{[MEDTA]}^{+n}}$ ?
Answer
569.7k+ views
Hint: EDTA is a polydentate ligand, which is more specifically a hexadentate ligand. The full form of EDTA is Ethylene diamine tetraacetic Acid. Hexadentate ligand means that EDTA has the capacity of having 6 bonding sites on its structure.
Complete answer:
EDTA is a hexadentate ligand which is able to donate electrons from six sites. These six sites are two nitrogen and four oxygen respectively. Here the ligand has a number of lone pair electrons to donate to the central atom. Many ligands are capable of binding metal by different sites because ligands have lone pair electrons on more than one atom.
After being bound into a metal complex the metal ions remain in the solutions but exhibit diminished reactivity. EDTA is produced as several salts as well.
The structure of ${{[MEDTA]}^{+n}}$ complex is:
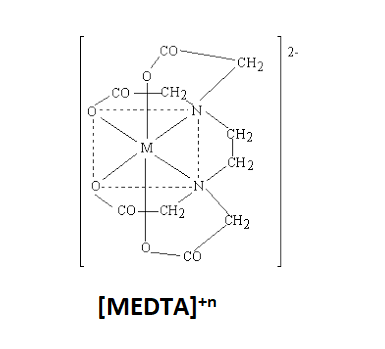
MEDTA complex is known as Metal Ethylene diamine tetraacetic Acid. The denticity of EDTA is 6. Hence, the number of chelate rings in EDTA will be one less than the denticity of EDTA. The number of chelate rings will be 5. So, this means that 5 chelate rings are present in the ${{[MEDTA]}^{+n}}$complex.
Note:
EDTA is a polyprotic acid which contains four carboxylic groups and also it contains two amine groups with lone pairs of electrons i.e. two on nitrogen and four on oxygen respectively. It is able to form stable complexes with water which leads to removal of hardness of water because the calcium and magnesium salts will react with the ligand and form complexes.
Complete answer:
EDTA is a hexadentate ligand which is able to donate electrons from six sites. These six sites are two nitrogen and four oxygen respectively. Here the ligand has a number of lone pair electrons to donate to the central atom. Many ligands are capable of binding metal by different sites because ligands have lone pair electrons on more than one atom.
After being bound into a metal complex the metal ions remain in the solutions but exhibit diminished reactivity. EDTA is produced as several salts as well.
The structure of ${{[MEDTA]}^{+n}}$ complex is:

MEDTA complex is known as Metal Ethylene diamine tetraacetic Acid. The denticity of EDTA is 6. Hence, the number of chelate rings in EDTA will be one less than the denticity of EDTA. The number of chelate rings will be 5. So, this means that 5 chelate rings are present in the ${{[MEDTA]}^{+n}}$complex.
Note:
EDTA is a polyprotic acid which contains four carboxylic groups and also it contains two amine groups with lone pairs of electrons i.e. two on nitrogen and four on oxygen respectively. It is able to form stable complexes with water which leads to removal of hardness of water because the calcium and magnesium salts will react with the ligand and form complexes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




