
How many right angles are there in a \[Xe{F_5}^ + \] ion?
Answer
490.5k+ views
Hint: Bases on the valence electrons of all atoms in a molecule, the molecular geometry and electron geometry will be expected. \[Xe{F_5}^ + \] has octahedral as electron geometry and square pyramidal as molecular geometry. Based on the molecular geometry, right angles can be calculated.
Complete answer:
\[Xe{F_5}^ + \] is a molecule consisting of Xenon as a central metal atom, and five fluorine atoms. The valence electrons on Xenon are \[8\] , and five fluorine atoms have \[5 \times 7 = 35\] electrons, as each fluorine atom has \[7\] valence electrons. Thus, the total valence electrons on the given molecule will be \[8 + 35 - 1 = 42\] electrons.
The eight electrons on a xenon atom are involved in bond formation with five fluorine atoms and one electron was lost as it is a cation, the remaining two electrons will exist as a lone pair of electrons. Thus, the hybridization is \[s{p^3}{d^2}\] . Thus, the electron geometry is octahedral, and the molecular geometry is square pyramidal due to the presence of one lone pair of electrons.
The structure of \[Xe{F_5}^ + \] is
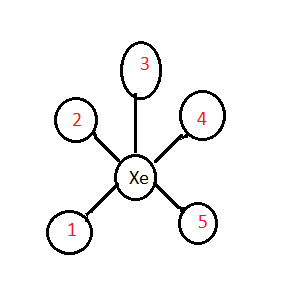
The five fluorine atoms were considered as \[1,2,3,4,\] and \[5\] . Based on the above structure the right angles were:
\[3 - Xe - 1\]
\[3 - Xe - 2\]
\[3 - Xe - 4\]
\[3 - Xe - 5\]
\[1 - Xe - 2\]
\[1 - Xe - 5\]
\[4 - Xe - 5\]
\[2 - Xe - 4\]
Thus, there is a total of eight right angles in \[Xe{F_5}^ + \]
Note:
Right angle means the angle must be equal to \[{90^0}\] . In the above structure, there were eight right angles, meaning there were eight bonds in which the bond angle equal to \[{90^0}\] . The right angles can be considered based on the molecular geometry but not electron geometry.
Complete answer:
\[Xe{F_5}^ + \] is a molecule consisting of Xenon as a central metal atom, and five fluorine atoms. The valence electrons on Xenon are \[8\] , and five fluorine atoms have \[5 \times 7 = 35\] electrons, as each fluorine atom has \[7\] valence electrons. Thus, the total valence electrons on the given molecule will be \[8 + 35 - 1 = 42\] electrons.
The eight electrons on a xenon atom are involved in bond formation with five fluorine atoms and one electron was lost as it is a cation, the remaining two electrons will exist as a lone pair of electrons. Thus, the hybridization is \[s{p^3}{d^2}\] . Thus, the electron geometry is octahedral, and the molecular geometry is square pyramidal due to the presence of one lone pair of electrons.
The structure of \[Xe{F_5}^ + \] is

The five fluorine atoms were considered as \[1,2,3,4,\] and \[5\] . Based on the above structure the right angles were:
\[3 - Xe - 1\]
\[3 - Xe - 2\]
\[3 - Xe - 4\]
\[3 - Xe - 5\]
\[1 - Xe - 2\]
\[1 - Xe - 5\]
\[4 - Xe - 5\]
\[2 - Xe - 4\]
Thus, there is a total of eight right angles in \[Xe{F_5}^ + \]
Note:
Right angle means the angle must be equal to \[{90^0}\] . In the above structure, there were eight right angles, meaning there were eight bonds in which the bond angle equal to \[{90^0}\] . The right angles can be considered based on the molecular geometry but not electron geometry.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




