
Rhombic sulphur has the following structure
(A) Open chain
(B) Tetrahedral
(C) Puckered 6 – membered ring
(D) Puckered 8 – membered ring
Answer
527.4k+ views
Hint: Sulphur in the form of rhombic sulphur is a crystalline allotropic form of the material. Sulphur in this form is the most stable. On standing, all other forms of sulphur ultimately return to rhombic form.
Complete answer:
-Open chain:
An open chain is a chain of atoms that is not joined at the ends and is thus reflected in its structural formula.
-Tetrahedral:
This molecule is made up of 4 sp3 hybrid orbitals that are evenly spaced and have bond angles of 109.5°. The orbitals have a tetrahedral form. Since each orbital has an atom at the centre, the molecule's form is tetrahedral.
-Puckered 6 – membered ring
The puckered 6-membered ring is a stable ring structure found in cycloalkanes (cyclohexane), and it is now referred to as an aromatic device.
-Puckered 8 – membered ring
Both naturally occurring elements would have an elemental form. Since it is the most stable structure and formula, this is the one the element would use the most.
In its elemental form, sulphur has an octatomic structure. This implies there are eight sulphur atoms arranged in a cyclic pattern.
Rhombic sulphur has a definite molecular structure. The S8 molecule is an 8-atom puckered ring with a 2.12A S-S distance.
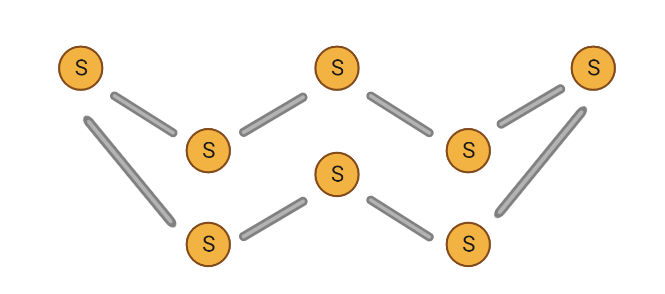
Thus, option (D) is the answer.
Note:
Powdered sulphur is dissolved in carbon disulphide at room temperature to make rhombic sulphur. After that, the mixture is filtered. The filtrate is then stored in a small beaker with filter paper on top. The carbon disulphide would slowly evaporate, leaving massive octahedral rhombic sulphur (or –sulphur) crystals behind.
Complete answer:
-Open chain:
An open chain is a chain of atoms that is not joined at the ends and is thus reflected in its structural formula.
-Tetrahedral:
This molecule is made up of 4 sp3 hybrid orbitals that are evenly spaced and have bond angles of 109.5°. The orbitals have a tetrahedral form. Since each orbital has an atom at the centre, the molecule's form is tetrahedral.
-Puckered 6 – membered ring
The puckered 6-membered ring is a stable ring structure found in cycloalkanes (cyclohexane), and it is now referred to as an aromatic device.
-Puckered 8 – membered ring
Both naturally occurring elements would have an elemental form. Since it is the most stable structure and formula, this is the one the element would use the most.
In its elemental form, sulphur has an octatomic structure. This implies there are eight sulphur atoms arranged in a cyclic pattern.
Rhombic sulphur has a definite molecular structure. The S8 molecule is an 8-atom puckered ring with a 2.12A S-S distance.

Thus, option (D) is the answer.
Note:
Powdered sulphur is dissolved in carbon disulphide at room temperature to make rhombic sulphur. After that, the mixture is filtered. The filtrate is then stored in a small beaker with filter paper on top. The carbon disulphide would slowly evaporate, leaving massive octahedral rhombic sulphur (or –sulphur) crystals behind.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




