
How many resonance structures exist for the formate ion, \[HC{{O}_{2}}^{-}\]?
Answer
564.3k+ views
Hint Formate is the anion i.e. negatively charged ion derived from formic acid. The IUPAC name of formate ion is methanoate and represented as\[HCO{{O}^{-}}\], \[CHO{{O}^{-}}\] and \[HC{{O}_{2}}^{-}\]. It is the product of deprotonation of formic acid.
Complete Step by step solution: Resonance structures are sets of Lewis structures which describe the delocalization of electrons in polyatomic ions or molecules. In many cases a single Lewis structure fails to explain the bonding in a molecule due to presence of partial charge and fractional bonds in it. In these cases resonance structure helps to describe the chemical bonding in that molecule.
The formate ion is composed of one atom of hydrogen and carbon and two atoms of oxygen in which the ion contains a charge of -1. It can be able to form resonance structures due to the presence of a lone pair in oxygen that can relocate or delocalize as a double bond between itself and the carbon atom which will cause the movement of the pi bond to move to the other oxygen atom as a lone pair. There are two resonance structure of formate ion are possible which can be shown as:
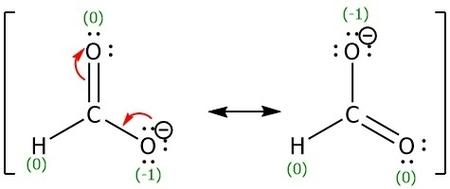
Negative charge is placed on the most electronegative element i.e. on oxygen.
Note: Resonance describes the bonding in particular molecules or ions by merging a number of contributory structures or forms which are jointly known as canonical structures or resonance structures within the theory of valence bonding into a hybrid resonance also known as hybrid structure.
Complete Step by step solution: Resonance structures are sets of Lewis structures which describe the delocalization of electrons in polyatomic ions or molecules. In many cases a single Lewis structure fails to explain the bonding in a molecule due to presence of partial charge and fractional bonds in it. In these cases resonance structure helps to describe the chemical bonding in that molecule.
The formate ion is composed of one atom of hydrogen and carbon and two atoms of oxygen in which the ion contains a charge of -1. It can be able to form resonance structures due to the presence of a lone pair in oxygen that can relocate or delocalize as a double bond between itself and the carbon atom which will cause the movement of the pi bond to move to the other oxygen atom as a lone pair. There are two resonance structure of formate ion are possible which can be shown as:

Negative charge is placed on the most electronegative element i.e. on oxygen.
Note: Resonance describes the bonding in particular molecules or ions by merging a number of contributory structures or forms which are jointly known as canonical structures or resonance structures within the theory of valence bonding into a hybrid resonance also known as hybrid structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




