
How do resonance structures affect bond length?
Answer
557.1k+ views
Hint: We must know that the bond length means distance in between the bonded two atoms in the structure. The bond length is dependent on the atomic radius of the two bonded atoms in the structure. Resonance is one of the important concepts in chemical bonding. If one structure has more resonance structure, it means that structure is more stable. Resonance energy means the energy difference between the conical structure and resonance hybrid structure.
Complete step by step answer:
As we know that the resonance hybrid structure means average of all the possible resonance structure in the molecule. If one structure is accepted to resonance means formation of that structure is nearly to our original structure. The average of all resonance structures is the main importance of the resonance concept. The bond length in the hybrid resonance structure is always an average of the bond length of all surrounding atoms and central atoms.
We explain, the resonance structure affects the bond length with an example of nitrate ion.
The formula of nitrate ion is $N{O_2}^ - $ . Nitrate ion having two resonance structures is possible. They are drawn below.
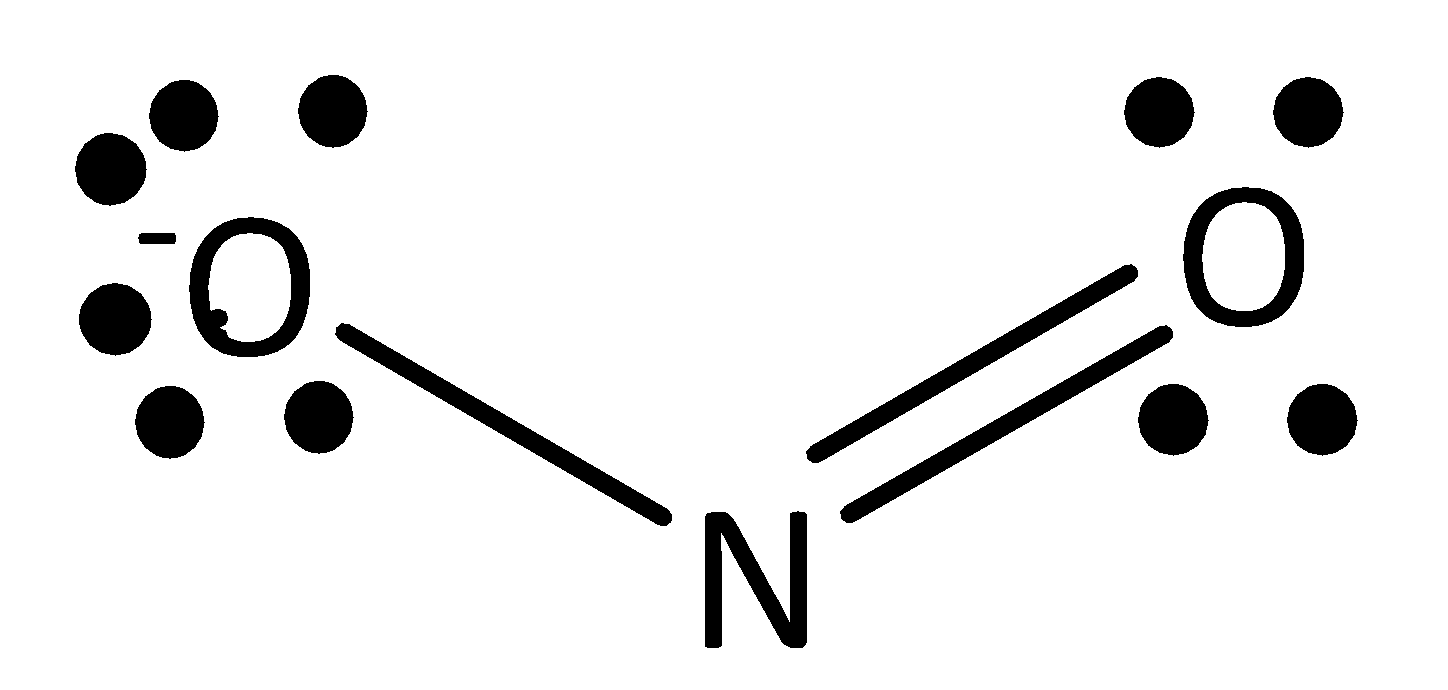
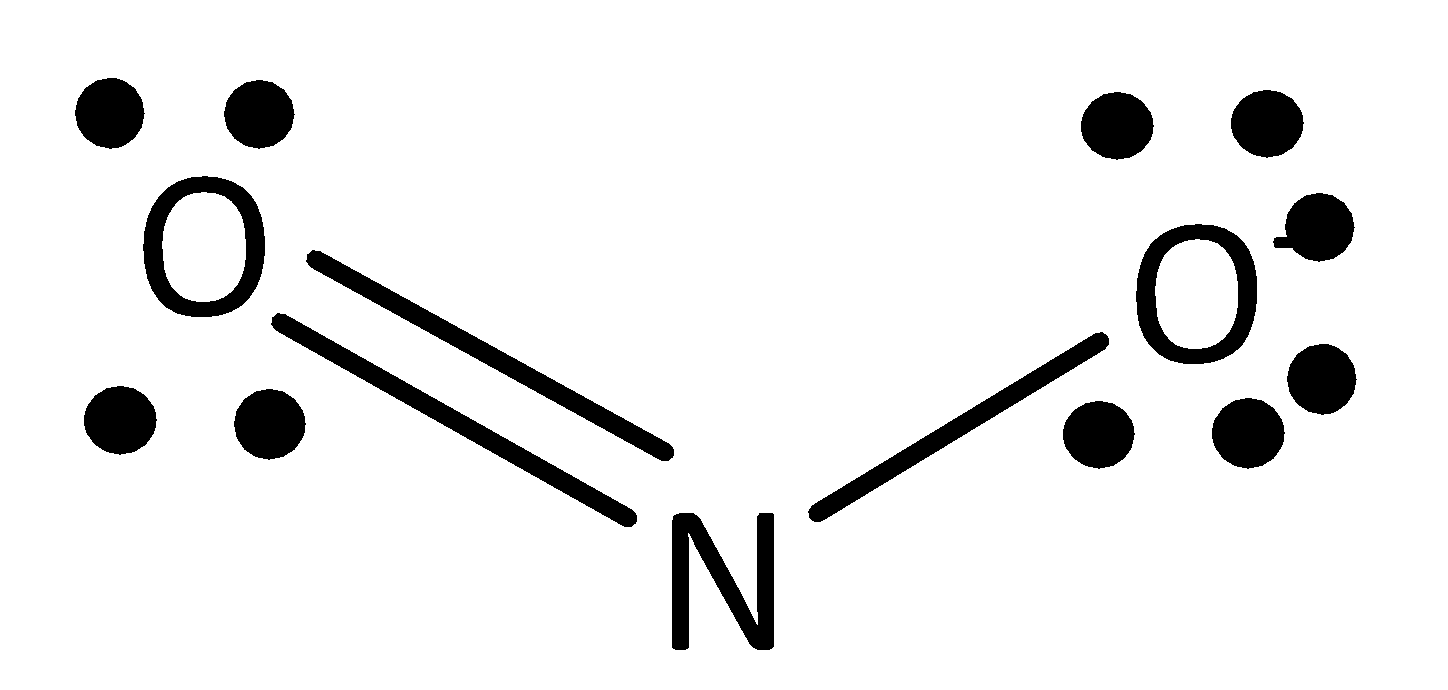
In both structures, nitrogen atoms have one single atom and double atom. This structure arises from the total valency of the structure $16$ . In this $16$ valence electrons, two electrons are involved in a single bond between nitrogen atoms and another four electrons are involved in a double bond between oxygen atom and nitrogen atom. Remaining ten electrons are in lone pairs of two oxygen atoms in structure.
The resonance hybrid structure of nitrate ion is given below,
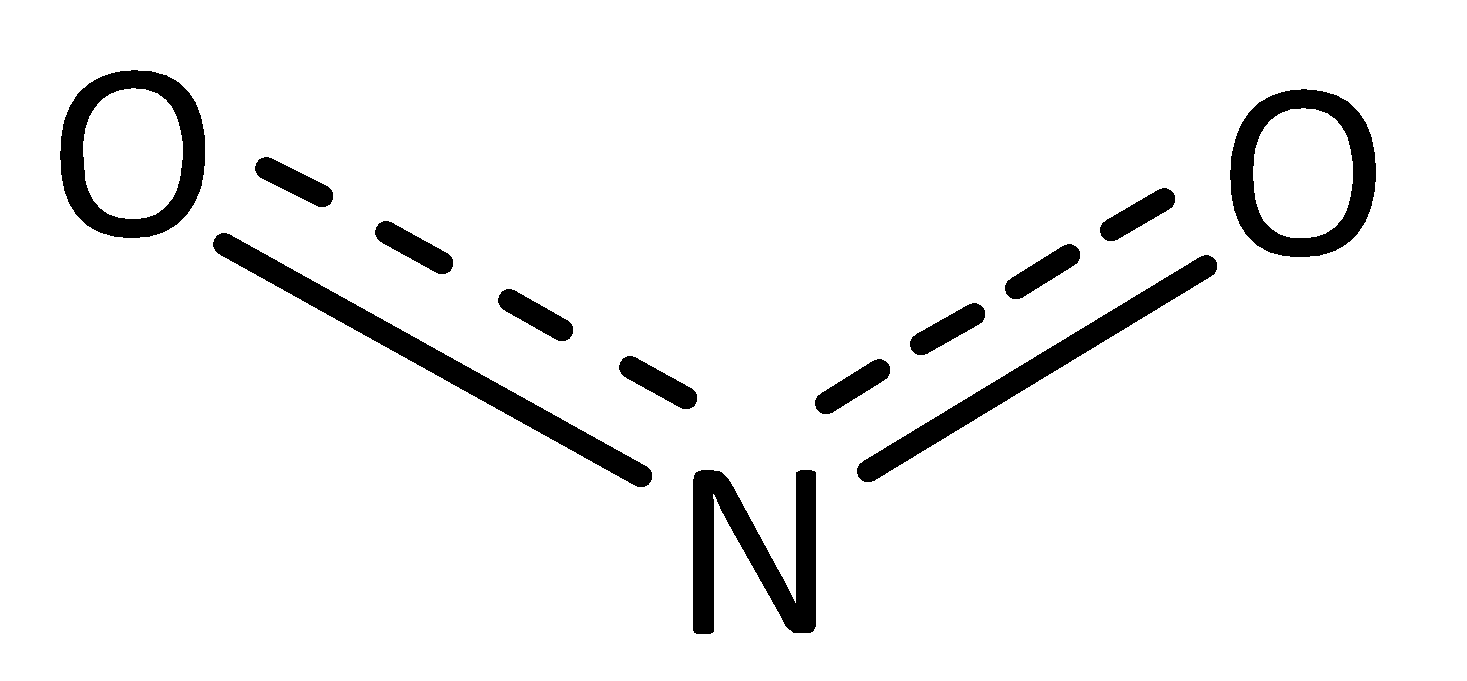
In this structure, the bond formed due to sharing a double bond and single bond between nitrogen and oxygen atoms. Hence, the average bond order of resonance hybrid structure in nitrate ions is $1.5$ . This bond is half of the single bond and double bond in the structure of nitrate ion. The original single bond length in nitrate $N - O$ is $136$ pm. The original double bond length in nitrate $N = O$ is $115$ pm. The average bond length of $N - O$ and $N = O$ is $125$ pm.
But, originally bond length in resonance hybrid structure in nitrate ion is $126$ pm.
In this way, resonance structure affects the bond length of the structure.
Note:
In resonance structure one important note is the charge of the central atom is never changed. The charge of the surrounding or bonded atom in the central atom is acceptable. The conical structure means the energy of the formation of that structure is nearly to our original structure of the molecule. The bond order means half of the difference between bonding electrons and anti bonding electrons in the structure. The bond order is one of the important factors to determine the bond stability in structure.
Complete step by step answer:
As we know that the resonance hybrid structure means average of all the possible resonance structure in the molecule. If one structure is accepted to resonance means formation of that structure is nearly to our original structure. The average of all resonance structures is the main importance of the resonance concept. The bond length in the hybrid resonance structure is always an average of the bond length of all surrounding atoms and central atoms.
We explain, the resonance structure affects the bond length with an example of nitrate ion.
The formula of nitrate ion is $N{O_2}^ - $ . Nitrate ion having two resonance structures is possible. They are drawn below.


In both structures, nitrogen atoms have one single atom and double atom. This structure arises from the total valency of the structure $16$ . In this $16$ valence electrons, two electrons are involved in a single bond between nitrogen atoms and another four electrons are involved in a double bond between oxygen atom and nitrogen atom. Remaining ten electrons are in lone pairs of two oxygen atoms in structure.
The resonance hybrid structure of nitrate ion is given below,

In this structure, the bond formed due to sharing a double bond and single bond between nitrogen and oxygen atoms. Hence, the average bond order of resonance hybrid structure in nitrate ions is $1.5$ . This bond is half of the single bond and double bond in the structure of nitrate ion. The original single bond length in nitrate $N - O$ is $136$ pm. The original double bond length in nitrate $N = O$ is $115$ pm. The average bond length of $N - O$ and $N = O$ is $125$ pm.
But, originally bond length in resonance hybrid structure in nitrate ion is $126$ pm.
In this way, resonance structure affects the bond length of the structure.
Note:
In resonance structure one important note is the charge of the central atom is never changed. The charge of the surrounding or bonded atom in the central atom is acceptable. The conical structure means the energy of the formation of that structure is nearly to our original structure of the molecule. The bond order means half of the difference between bonding electrons and anti bonding electrons in the structure. The bond order is one of the important factors to determine the bond stability in structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




