
How do resonance structure and isomers differ?
Answer
478.5k+ views
Hint: In order to answer this question we should know about the resonance structure and isomers. The difference in the location of electrons happens in the resonance while the difference in the location of atoms in the isomers.
Complete answer:
The difference between Resonance structure and isomers are as follows:
Addition information:
Resonance structures are very useful when a structure of a single molecule is unable to describe the bonding which is occurring between neighboring atoms. Whereas Isomers are useful as the two isomers have an identical chemical formula but their chemical properties and structures are not the same. The structure of the molecule contributes to its properties.
Note:
The resonance structure can form when there are two or more than two ways by which we can draw a Lewis dot diagram that satisfies the octet rule. Thus those Lewis structures which are equivalent are termed resonance structures.
Complete answer:
The difference between Resonance structure and isomers are as follows:
| Resonance Structure | Isomers |
Resonance structures are those in which more than one Lewis dot structure can be drawn.In resonance, the resonance structures are of the same compound.The resonance structures tell us about the delocalization of electrons present in the polyatomic ion or a molecule.Example: There are two resonance contributors in acetone. 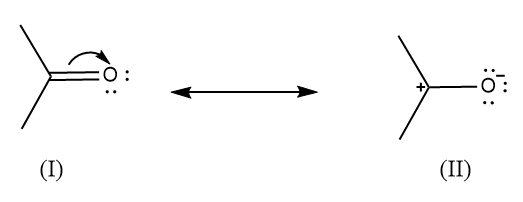
| Isomers are those chemical compounds that have the same chemical formula but different in properties. As there are arrangements of atoms, the isomers are different compounds.Isomers have a different arrangement of atoms in the molecule.Example: Dimethyl ether and ethanol are isomers. 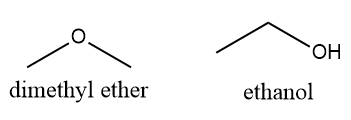
|
Addition information:
Resonance structures are very useful when a structure of a single molecule is unable to describe the bonding which is occurring between neighboring atoms. Whereas Isomers are useful as the two isomers have an identical chemical formula but their chemical properties and structures are not the same. The structure of the molecule contributes to its properties.
Note:
The resonance structure can form when there are two or more than two ways by which we can draw a Lewis dot diagram that satisfies the octet rule. Thus those Lewis structures which are equivalent are termed resonance structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




