
What is regioselectivity and stereoselectivity?
Answer
536.4k+ views
Hint: Many chemical reactions involve the transformations of a reactant to multiple isomeric products. The preference for one product over another is known as selectivity. Regioselectivity and stereoselectivity are related to the formation of a selective isomer over another.
Complete answer:
The addition of a molecule to the reactant species having more than one attacking site can result in the formation of more than one product. The products can be isomeric with each other. But experimentally it was found that in such reactions, the formation of one product dominates over the another. This preferential formation of products is known as selectivity.
The reaction in which the formation of one constitutional isomer over another is known as regioselective reaction and this property of bonding at a particular site of a molecule to yield one of the isomers in a larger amount is known as regioselectivity.
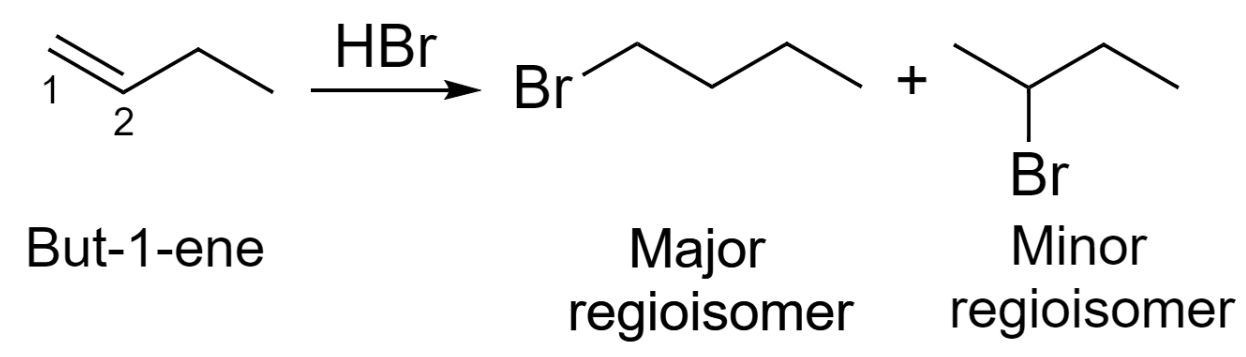
Markovnikov additions are common examples of regioselective reactions. In addition to HBr to but-1-ene, we would see a preference for the formation of a secondary carbocation over primary carbocation, though it would seem either orientation is possible.
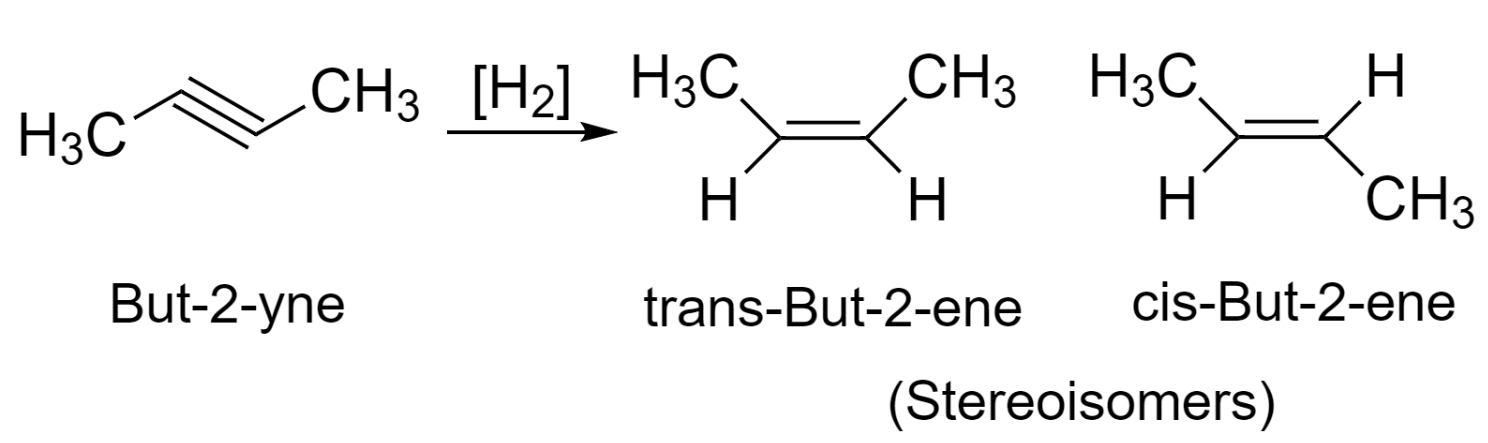
The reaction is said to be stereoselective when a reactant yields more than one stereoisomer product under the same reaction conditions and one of the products formed is in the larger amount. It is concerned with what conformation the products will be after the reaction.
A basic example of this is ${{\text{S}}_{\text{N}}}\text{2}$ reactions as given below.
Note:
A reaction in which the stereochemistry of the reactants decides the outcome of the reaction and produces a specific product is known as a stereospecific reaction. In simple words, stereoselectivity means preferential product formation over the others while stereospecificity means specific stereoisomers of reactant forms specific products.
Complete answer:
The addition of a molecule to the reactant species having more than one attacking site can result in the formation of more than one product. The products can be isomeric with each other. But experimentally it was found that in such reactions, the formation of one product dominates over the another. This preferential formation of products is known as selectivity.
The reaction in which the formation of one constitutional isomer over another is known as regioselective reaction and this property of bonding at a particular site of a molecule to yield one of the isomers in a larger amount is known as regioselectivity.
Markovnikov additions are common examples of regioselective reactions. In addition to HBr to but-1-ene, we would see a preference for the formation of a secondary carbocation over primary carbocation, though it would seem either orientation is possible.

The reaction is said to be stereoselective when a reactant yields more than one stereoisomer product under the same reaction conditions and one of the products formed is in the larger amount. It is concerned with what conformation the products will be after the reaction.
A basic example of this is ${{\text{S}}_{\text{N}}}\text{2}$ reactions as given below.
Note:
A reaction in which the stereochemistry of the reactants decides the outcome of the reaction and produces a specific product is known as a stereospecific reaction. In simple words, stereoselectivity means preferential product formation over the others while stereospecificity means specific stereoisomers of reactant forms specific products.

Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




