
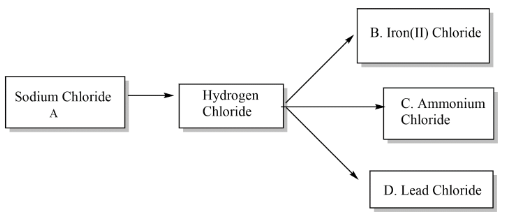
Refer to the flow chart diagram below and give balanced equations with conditions, if any for the following conversion A to D.

Answer
547.5k+ views
Hint:The conversion of some substance from one form to another can be done in laboratories and also in industries. So many named reactions as well as the named processes are used. In the given diagram or flowchart the conversion of sodium chloride to hydrogen chloride is an industrial step while after which other products are formed from it.
Complete step-by-step answer:The hydrogen chloride can be written as by the reaction between hydrogen gas and chlorine gas,
\[{H_2}\, + C{l_2} \to \,2HCl\] we can prepare the hydrochloride in lab by direct combination of both the gases but the production of hydrogen chloride from sodium chloride takes place at industries as large scale preparation. In industrial scale, reaction of sodium chloride takes place with sulfuric acid. We can take in place of sodium chloride many chlorides like $PC{l_3}\,,\,SOC{l_2}$ . $PC{l_3}$ is phosphorus trichloride while $SOC{l_2}$ is thionyl chloride, after chlorination a by-product is obtained which is benzene. So, we can write the reaction as follow,
$NaCl\,(A)\,\, + \,{H_2}S{O_4}\, \to \,NaHS{O_4}\, + HCl$
This reaction takes place at room temperature, now the conversions are seen from $HCl$ to iron chloride, ammonium chloride and lead chloride. $HCl$ Hydrogen chloride reacts with iron to give iron chloride and hydrogen gas is evolved. This can be written in the form of reaction and we get our compound (B)
$2HCl + \,Fe\, \to FeC{l_2}\,(B)\, + \,{H_2}$
Next step is to see how hydrogen chloride converts to ammonium chloride, for this reaction ammonia gas is passed through the hydrogen chloride solution. It was seen that this reaction is reversible; it means the rate of formation of ammonium chloride is equal to the rate of decomposition of it. There are many reaction of ammonium salts which are reversible hence for this reaction the pressure should be high. By the high pressure the equilibrium will shift towards forward reaction and hence greater yield will obtain. This concept is according to the Le’ Chatelier principle and we get compound (C)
$N{H_3}\,(g) + \,HCl(g) \rightleftharpoons \,N{H_4}Cl(s)\,\,(C)$
Last we have to convert hydrogen chloride into lead chloride, for this conversion we need lead which will reacts with hydrogen chloride and gives lead chloride which is compound (D). If we write the reaction between them we can say that a solid lead will reacts with dilute or concentrated solution of hydrogen chloride and give $PbC{l_2}$
$Pb(s)\, + \,2HCl(aq)\, \to \,PbC{l_2}(aq)\,\,(D) + {H_2}(g)$
Hence like this all compounds are converted from hydrogen chloride.
Note: There are two methods for preparation of lead chloride from dilute solution as well as concentrated solution of hydrogen chloride it was governed using solubility coefficient. Also, the reaction which takes place at equilibrium will need a change to balance the factors which get disturb during equilibrium.
Complete step-by-step answer:The hydrogen chloride can be written as by the reaction between hydrogen gas and chlorine gas,
\[{H_2}\, + C{l_2} \to \,2HCl\] we can prepare the hydrochloride in lab by direct combination of both the gases but the production of hydrogen chloride from sodium chloride takes place at industries as large scale preparation. In industrial scale, reaction of sodium chloride takes place with sulfuric acid. We can take in place of sodium chloride many chlorides like $PC{l_3}\,,\,SOC{l_2}$ . $PC{l_3}$ is phosphorus trichloride while $SOC{l_2}$ is thionyl chloride, after chlorination a by-product is obtained which is benzene. So, we can write the reaction as follow,
$NaCl\,(A)\,\, + \,{H_2}S{O_4}\, \to \,NaHS{O_4}\, + HCl$
This reaction takes place at room temperature, now the conversions are seen from $HCl$ to iron chloride, ammonium chloride and lead chloride. $HCl$ Hydrogen chloride reacts with iron to give iron chloride and hydrogen gas is evolved. This can be written in the form of reaction and we get our compound (B)
$2HCl + \,Fe\, \to FeC{l_2}\,(B)\, + \,{H_2}$
Next step is to see how hydrogen chloride converts to ammonium chloride, for this reaction ammonia gas is passed through the hydrogen chloride solution. It was seen that this reaction is reversible; it means the rate of formation of ammonium chloride is equal to the rate of decomposition of it. There are many reaction of ammonium salts which are reversible hence for this reaction the pressure should be high. By the high pressure the equilibrium will shift towards forward reaction and hence greater yield will obtain. This concept is according to the Le’ Chatelier principle and we get compound (C)
$N{H_3}\,(g) + \,HCl(g) \rightleftharpoons \,N{H_4}Cl(s)\,\,(C)$
Last we have to convert hydrogen chloride into lead chloride, for this conversion we need lead which will reacts with hydrogen chloride and gives lead chloride which is compound (D). If we write the reaction between them we can say that a solid lead will reacts with dilute or concentrated solution of hydrogen chloride and give $PbC{l_2}$
$Pb(s)\, + \,2HCl(aq)\, \to \,PbC{l_2}(aq)\,\,(D) + {H_2}(g)$
Hence like this all compounds are converted from hydrogen chloride.
Note: There are two methods for preparation of lead chloride from dilute solution as well as concentrated solution of hydrogen chloride it was governed using solubility coefficient. Also, the reaction which takes place at equilibrium will need a change to balance the factors which get disturb during equilibrium.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Which Country is Called "The Land of Festivals"?

What type of cell is found in the Seminiferous tub class 10 biology CBSE

What are the public facilities provided by the government? Also explain each facility




