
Reduction of nitrobenzene in the presence of Zn \[N{{H}_{4}}Cl\] gives:
A) Hydrazobenzene
B) Aniline
C) Azobenzene
D) N-phenylhydroxylamine
Answer
604.5k+ views
Hint: Ammonium salts, such as ammonium formate and acetate, can also be used as a promoter for zinc reduction. Promoters, like ammonium chloride, serve as electrolytes and complex forming agents to enable zinc reduction of nitrobenzene to proceed at near ambient temperatures.
Complete answer:
For reduction of a nitro compound into an amine, it requires six units of reducing agent.
\[R-N{{O}_{2}}+6{{H}^{+}}+6{{e}^{-}}\to RN{{H}_{2}}+2{{H}_{2}}O\]
The whole reaction doesn’t occur in a single step, instead it requires multiple steps with forming a chain of intermediates, which with strong reducing agents in acidic solution have a small transient existence. Intermediates formed by adding two units of reducing agents to \[RN{{O}_{2}}\] are nitroso compounds, \[RN=O\]and N-substituted hydroxylamines \[RNHOH\].
\[RN{{O}_{2}}\xrightarrow[-{{H}_{2}}O]{2[H]}RN=O\xrightarrow{2[H]}RNHOH\xrightarrow[-{{H}_{2}}O]{2[H]}RN{{H}_{2}}\]
The n- substituted hydroxylamine can be directly obtained from the respective nitro compounds with Zn and \[N{{H}_{4}}Cl\].
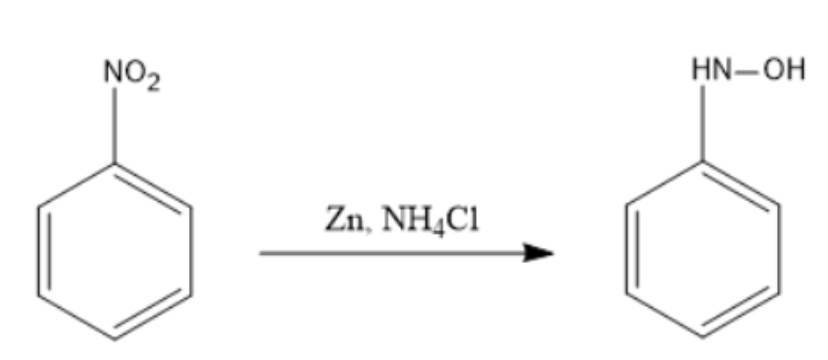
Reduction of nitrobenzene with the help of Zn is an electrolytic reaction done in the aqueous phase, where an electrolyte is required to serve as the promoter. Generally, ammonium chloride \[N{{H}_{4}}Cl\] is used as a promoter and the reaction is carried out at a temperature of 50℃– 65℃.
The exact mechanism for the formation of N-substituted hydroxylamine can be seen below.
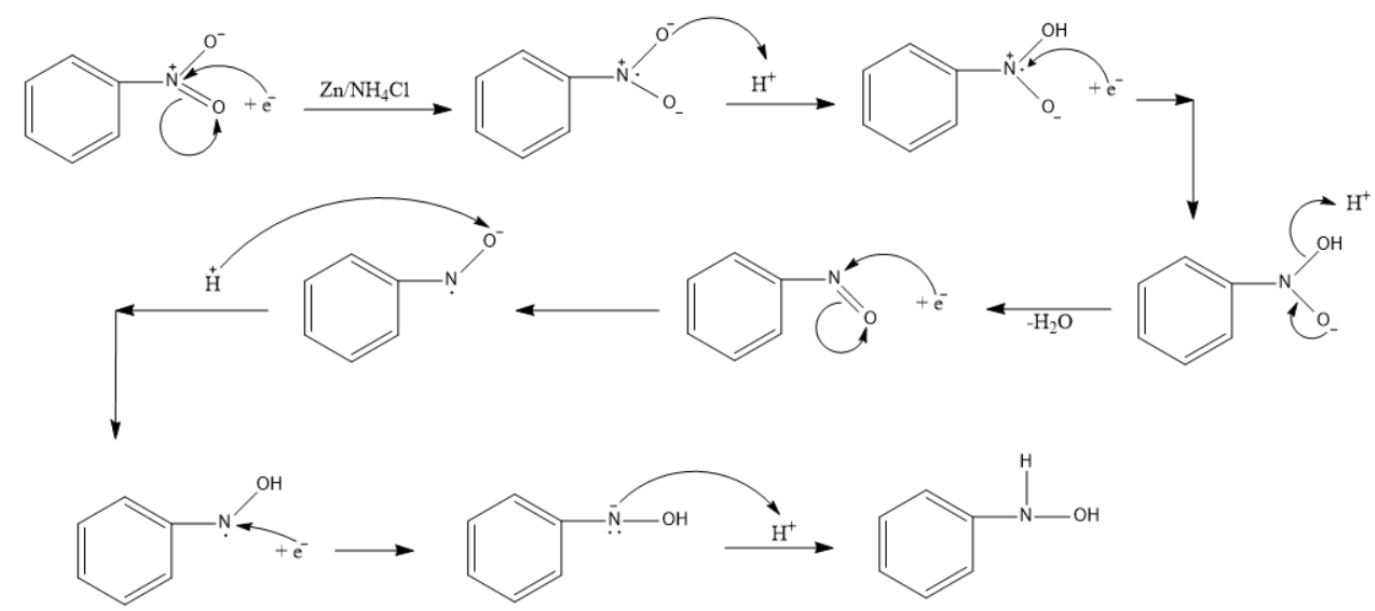
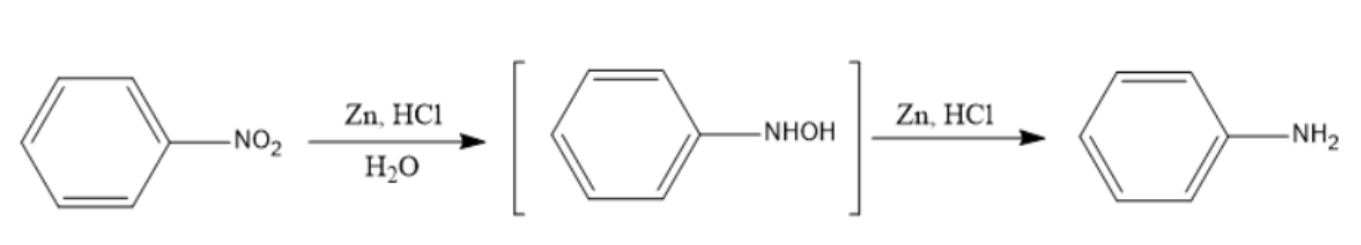
Note: Under mildly alkaline conditions, the reduction is terminated at the formation of N-substituted hydroxylamine. But under acidic conditions, the reaction proceeds further to form aniline as seen in the picture below. In the second branch we have used Zn and HCl instead of Zn and \[N{{H}_{4}}Cl\].
Complete answer:
For reduction of a nitro compound into an amine, it requires six units of reducing agent.
\[R-N{{O}_{2}}+6{{H}^{+}}+6{{e}^{-}}\to RN{{H}_{2}}+2{{H}_{2}}O\]
The whole reaction doesn’t occur in a single step, instead it requires multiple steps with forming a chain of intermediates, which with strong reducing agents in acidic solution have a small transient existence. Intermediates formed by adding two units of reducing agents to \[RN{{O}_{2}}\] are nitroso compounds, \[RN=O\]and N-substituted hydroxylamines \[RNHOH\].
\[RN{{O}_{2}}\xrightarrow[-{{H}_{2}}O]{2[H]}RN=O\xrightarrow{2[H]}RNHOH\xrightarrow[-{{H}_{2}}O]{2[H]}RN{{H}_{2}}\]
The n- substituted hydroxylamine can be directly obtained from the respective nitro compounds with Zn and \[N{{H}_{4}}Cl\].

Reduction of nitrobenzene with the help of Zn is an electrolytic reaction done in the aqueous phase, where an electrolyte is required to serve as the promoter. Generally, ammonium chloride \[N{{H}_{4}}Cl\] is used as a promoter and the reaction is carried out at a temperature of 50℃– 65℃.
The exact mechanism for the formation of N-substituted hydroxylamine can be seen below.

Note: Under mildly alkaline conditions, the reduction is terminated at the formation of N-substituted hydroxylamine. But under acidic conditions, the reaction proceeds further to form aniline as seen in the picture below. In the second branch we have used Zn and HCl instead of Zn and \[N{{H}_{4}}Cl\].

Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

How is the angle of emergence e related to the angle class 12 physics CBSE

Differentiate between lanthanoids and actinoids class 12 chemistry CBSE

Derive Lens Makers formula for a convex lens class 12 physics CBSE

a Draw Labelled diagram of Standard Hydrogen Electrode class 12 chemistry CBSE




