
Reduction of aromatic nitro compounds using Fe and HCL gives
a. aromatic oxime
b. aromatic hydrocarbon
c. aromatic primary amine
d. aromatic amide
Answer
576k+ views
Hint: In the given question reduction of aromatic nitro compounds in presence of iron and hydrochloric acid is explained. Aromatic nitro compounds are typically synthesized by the nitration process. It is achieved by using a combination of nitric acid and sulfuric acid, which yield the nitronium ion \[\left( {N{O_{ 2}^+}} \right)\]
Complete Step by step answer:
As we know that the nitro group is one of the first functional groups which can be reduced. The Nitro compounds are organic compounds which comprise of one or more than one nitro functional groups \[\left( { - N{O_2}} \right)\]. The nitro group is one of the most common explosophores that is the functional group which makes a compound explosive and is used worldwide in making explosives. The nitro group is also strongly electron-withdrawing group thus the Reduction of nitro aromatic compounds in presence of iron and hydrochloric acid gives aromatic primary amines.
The Nitro compounds participate in several organic reactions, the most important thing to note is their reduction to the corresponding amines thus the reaction occur is as follows:
\[R - N{O_2} + {\text{ }}3{H_2}\xrightarrow{{Fe/HCl}}R - N{H_2} + {\text{ }}2{H_2}O\]
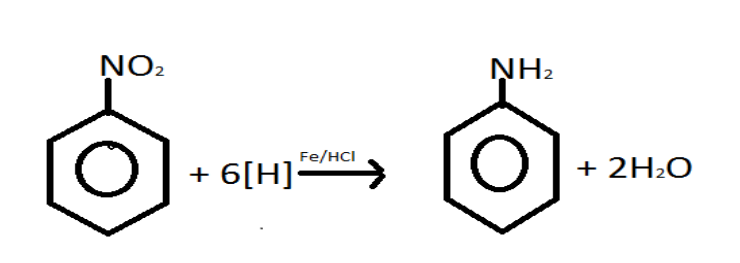
Thus, option \[C\] is correct.
Note: In organic chemistry the chemical reactions of reduction of nitro compounds are of wide interest worldwide. On a large industrial scale, the reduction of nitroaromatics has been conducted by using the method of Catalytic hydrogenation, where hydrogenation is a chemical reaction which occur in between molecular hydrogen \[\left( {H2} \right)\] and another element, usually in the presence of a catalyst such as palladium.
Complete Step by step answer:
As we know that the nitro group is one of the first functional groups which can be reduced. The Nitro compounds are organic compounds which comprise of one or more than one nitro functional groups \[\left( { - N{O_2}} \right)\]. The nitro group is one of the most common explosophores that is the functional group which makes a compound explosive and is used worldwide in making explosives. The nitro group is also strongly electron-withdrawing group thus the Reduction of nitro aromatic compounds in presence of iron and hydrochloric acid gives aromatic primary amines.
The Nitro compounds participate in several organic reactions, the most important thing to note is their reduction to the corresponding amines thus the reaction occur is as follows:
\[R - N{O_2} + {\text{ }}3{H_2}\xrightarrow{{Fe/HCl}}R - N{H_2} + {\text{ }}2{H_2}O\]

Thus, option \[C\] is correct.
Note: In organic chemistry the chemical reactions of reduction of nitro compounds are of wide interest worldwide. On a large industrial scale, the reduction of nitroaromatics has been conducted by using the method of Catalytic hydrogenation, where hydrogenation is a chemical reaction which occur in between molecular hydrogen \[\left( {H2} \right)\] and another element, usually in the presence of a catalyst such as palladium.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




