
Reactions of deuterated bromocyclohexene, 1 and 2, by the E2 mechanism give mono deuterated cyclohexene A and d'deuterated cyclohexene B. Which of the statements (a)-(b) best indicates the most probable selectivities of two reactions.
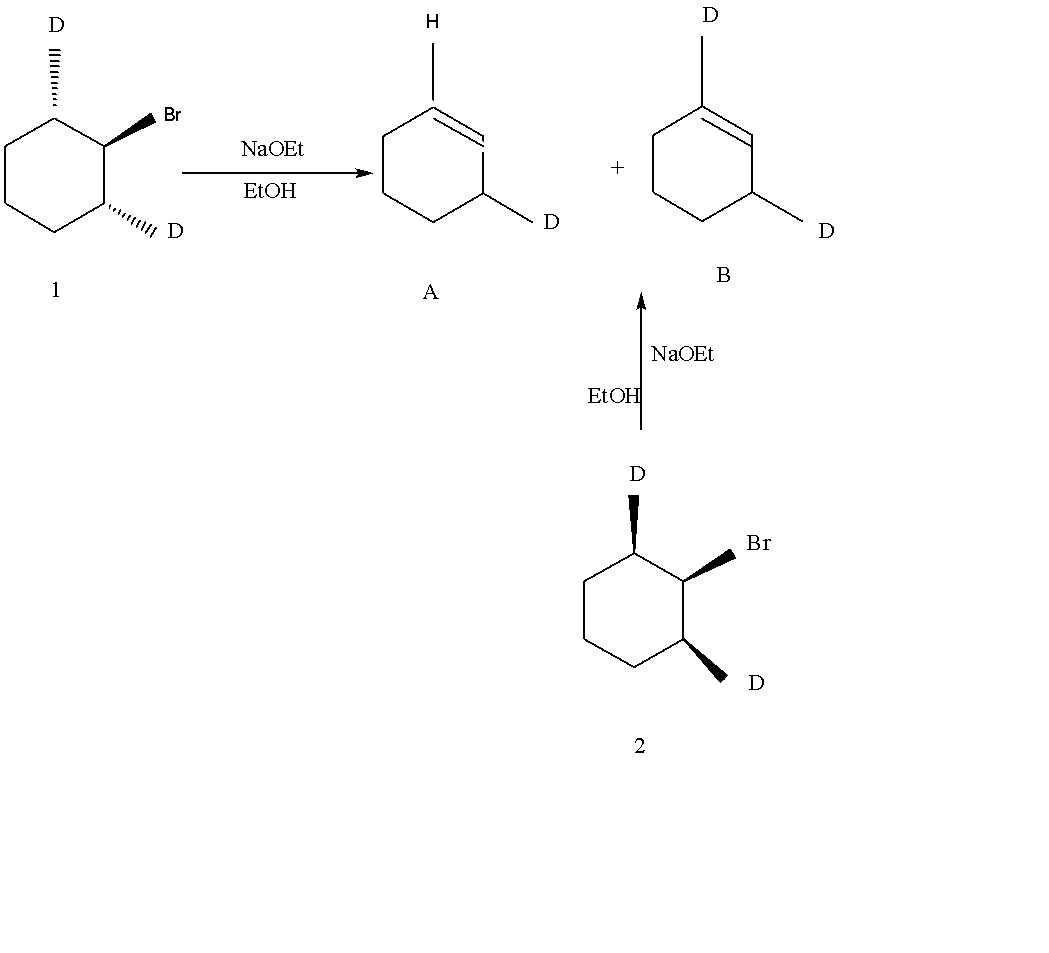
A.Product is A form both 1 and 2.
B.Product is B from both 1 and 2.
C.Product is A from 1 and B from 2.
D.Product is B from 1 and A from 2.

Answer
569.4k+ views
Hint: Elimination reactions can be very useful in organic synthesis for producing alkenes, alkynes and allenes on a preexisting scaffold. Synthetic issues associated with eliminations include the suppression of substitution and suitability in synthesis will be discussed and methods involving stereo selective and stereospecific transformation to di, tri, and tetra substituted alkenes will be disclosed. Elimination reactions that form alkynes and both enantioenriched and racemic allenes are also featured.
Complete answer:
Elimination reactions are commonly known by the kind of atoms or groups of atoms leaving the molecules. The removal of a hydrogen atom and halogen atom. E reaction occurs by elimination reaction and facilitated. When the H and nucleofuge on adjacent Carbon atoms can achieve an antiplanar. When the 1 with the Bromine axial, the trans C-D bonds are both axial and coplanar with the bromine. Elimination of D with Bromine gives yield A by the E2 mechanism. As it is, the conformation of 2 with the Br axial, two adjacent are in trans position. Here C-H bonds are in axial and coplanar with the Br and give yield B by the E2 mechanism.
So, Option C is the correct answer.
Note: E2 reactions are typically seen with secondary and tertiary alkyl halides, but a hindered base is necessary with a primary halide. The mechanism by which it occurs is a single step concerted reaction with one transition state. The rate at which this mechanism occurs is second order kinetics, and depends on both the base and alkyl halide.
Complete answer:
Elimination reactions are commonly known by the kind of atoms or groups of atoms leaving the molecules. The removal of a hydrogen atom and halogen atom. E reaction occurs by elimination reaction and facilitated. When the H and nucleofuge on adjacent Carbon atoms can achieve an antiplanar. When the 1 with the Bromine axial, the trans C-D bonds are both axial and coplanar with the bromine. Elimination of D with Bromine gives yield A by the E2 mechanism. As it is, the conformation of 2 with the Br axial, two adjacent are in trans position. Here C-H bonds are in axial and coplanar with the Br and give yield B by the E2 mechanism.
So, Option C is the correct answer.
Note: E2 reactions are typically seen with secondary and tertiary alkyl halides, but a hindered base is necessary with a primary halide. The mechanism by which it occurs is a single step concerted reaction with one transition state. The rate at which this mechanism occurs is second order kinetics, and depends on both the base and alkyl halide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




