
Reaction of t-butyl bromide with sodium methoxide produces:
(A) Isobutane
(B) Isobutylene
(C) Sodium- t- butoxide
(D) t- butyl methyl ether.
Answer
587.1k+ views
Hint: This method is called Williamson’s synthesis. General reaction of this synthesis is:
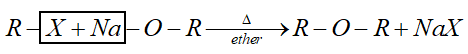
Complete step by step answer:
When alkyl halide is heated with alc. Sodium or potassium alkoxide gives corresponding ethers. Simple ethers can be easily prepared by this method.
But for preparation of mixed ether a proper choice of reactant is necessary. Primary alkyl halide is more susceptible to $S{N^2}$ reaction. Therefore, best yield of ether obtained when alkyl halide is primary and alkoxide is tertiary.
Example: tert-butyl ether is prepared by heating ethyl bromide with sodium tert-butoxide.
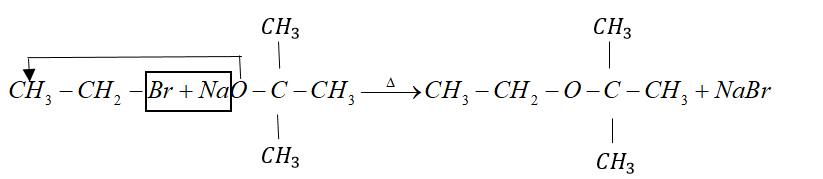
But when alkyl halide is secondary or tertiary the nucleophilic attack of alkoxide ion on $\alpha - $ carbon atom becomes difficult due to crowding effect.
Since alkoxide is a stronger base and attacking $\beta - $ hydrogen is easier. Therefore $\beta - $ elimination dominates.
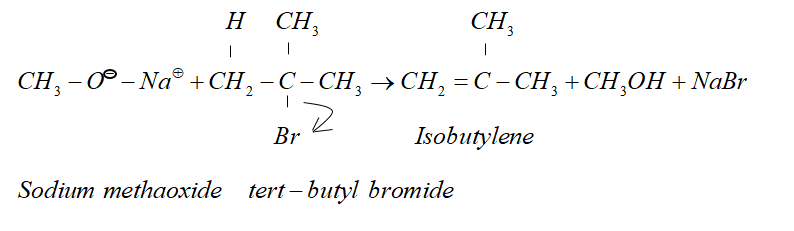
Therefore, in the given reaction isobutylene is formed.
So, the correct answer is “Option B”.
Note:
Williamson’s synthesis is a good method for preparation of mixed ether. But proper choice of reagent should be necessary. If we want to prepare t-butyl methyl ether then methyl bromide and sodium t-butoxide should be taken.
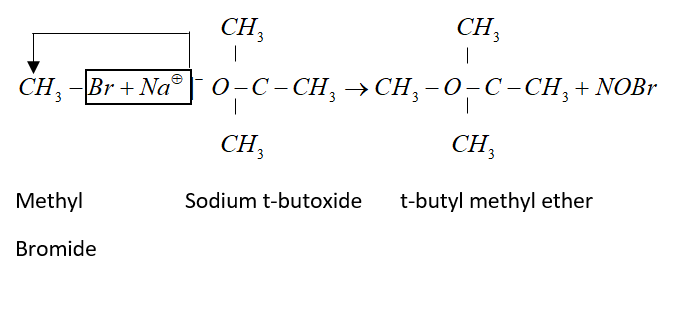
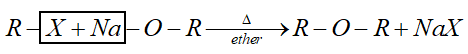
Complete step by step answer:
When alkyl halide is heated with alc. Sodium or potassium alkoxide gives corresponding ethers. Simple ethers can be easily prepared by this method.
But for preparation of mixed ether a proper choice of reactant is necessary. Primary alkyl halide is more susceptible to $S{N^2}$ reaction. Therefore, best yield of ether obtained when alkyl halide is primary and alkoxide is tertiary.
Example: tert-butyl ether is prepared by heating ethyl bromide with sodium tert-butoxide.
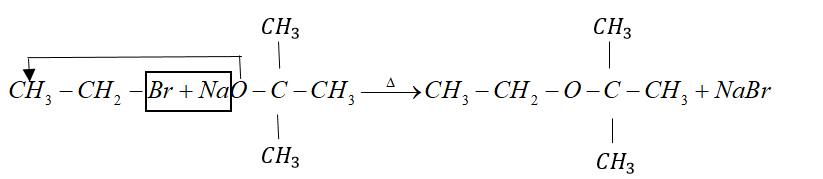
But when alkyl halide is secondary or tertiary the nucleophilic attack of alkoxide ion on $\alpha - $ carbon atom becomes difficult due to crowding effect.
Since alkoxide is a stronger base and attacking $\beta - $ hydrogen is easier. Therefore $\beta - $ elimination dominates.
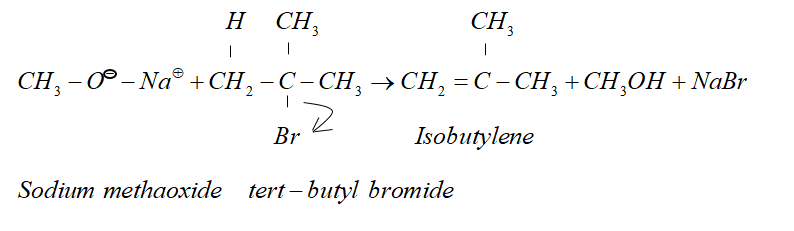
Therefore, in the given reaction isobutylene is formed.
So, the correct answer is “Option B”.
Note:
Williamson’s synthesis is a good method for preparation of mixed ether. But proper choice of reagent should be necessary. If we want to prepare t-butyl methyl ether then methyl bromide and sodium t-butoxide should be taken.

Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




