
Reaction of iron nails with copper sulphate solution is an example of:
A. Combination
B. Decomposition
C. Displacement reaction
D. Double displacement reaction
Answer
520.2k+ views
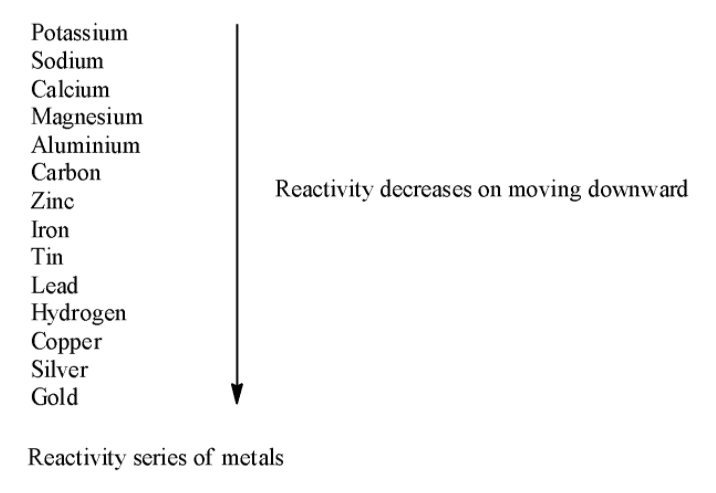
Hint: We know that a reaction where displacement of a less reactive metal from its compound occurs by a less reactive metal is termed as displacement reaction. The reactivity of metals is decided by the reactivity series of metals.
Complete step by step answer:
From the reactivity series of metals we know that iron is more reactive than copper. So, iron displaces copper from copper sulphate. The reaction of iron and copper sulphate can be shown as follows:
${\rm{Fe}} + {\rm{CuS}}{{\rm{O}}_{\rm{4}}} \to {\rm{FeS}}{{\rm{O}}_{\rm{4}}} + {\rm{Cu}}$
So, reaction of copper sulphate with iron is an example of displacement reaction.
Hence, option C is correct.
Additional Information:
Combination reaction is the reaction in which two or more reactants combine to form a product.
One example of combination reaction is formation of water from oxygen and hydrogen.
${\rm{2}}{{\rm{H}}_{\rm{2}}} + 2{{\rm{O}}_{\rm{2}}} \to 2{{\rm{H}}_{\rm{2}}}{\rm{O}}$
Decomposition reaction is the reaction in which a reactant decomposes to produce two or more products. One example of decomposition reaction is,
${\rm{A}}{{\rm{l}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}} \to {\rm{Al}} + {{\rm{O}}_{\rm{2}}}$
In a double displacement reaction, the exchange of ions of two ionic compounds takes place. The reaction between potassium nitrate and aluminium chloride represents a double displacement reaction.
${\rm{KN}}{{\rm{O}}_{\rm{3}}} + {\rm{AlC}}{{\rm{l}}_{\rm{3}}} \to {\rm{Al}}{\left( {{\rm{N}}{{\rm{O}}_{\rm{3}}}} \right)_3} + {\rm{KCl}}$
Note:
Activity series of metals is a list of metals arranged according to their reactivity. The highly reactive metal displaces less reactive metal in displacement reaction. As potassium is the highest reactive metal it can displace all other metal atoms from their compounds. Similarly, zinc can displace iron from iron sulphate as zinc is more reactive than iron.
Complete step by step answer:
From the reactivity series of metals we know that iron is more reactive than copper. So, iron displaces copper from copper sulphate. The reaction of iron and copper sulphate can be shown as follows:
${\rm{Fe}} + {\rm{CuS}}{{\rm{O}}_{\rm{4}}} \to {\rm{FeS}}{{\rm{O}}_{\rm{4}}} + {\rm{Cu}}$
So, reaction of copper sulphate with iron is an example of displacement reaction.
Hence, option C is correct.
Additional Information:
Combination reaction is the reaction in which two or more reactants combine to form a product.
One example of combination reaction is formation of water from oxygen and hydrogen.
${\rm{2}}{{\rm{H}}_{\rm{2}}} + 2{{\rm{O}}_{\rm{2}}} \to 2{{\rm{H}}_{\rm{2}}}{\rm{O}}$
Decomposition reaction is the reaction in which a reactant decomposes to produce two or more products. One example of decomposition reaction is,
${\rm{A}}{{\rm{l}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}} \to {\rm{Al}} + {{\rm{O}}_{\rm{2}}}$
In a double displacement reaction, the exchange of ions of two ionic compounds takes place. The reaction between potassium nitrate and aluminium chloride represents a double displacement reaction.
${\rm{KN}}{{\rm{O}}_{\rm{3}}} + {\rm{AlC}}{{\rm{l}}_{\rm{3}}} \to {\rm{Al}}{\left( {{\rm{N}}{{\rm{O}}_{\rm{3}}}} \right)_3} + {\rm{KCl}}$
Note:
Activity series of metals is a list of metals arranged according to their reactivity. The highly reactive metal displaces less reactive metal in displacement reaction. As potassium is the highest reactive metal it can displace all other metal atoms from their compounds. Similarly, zinc can displace iron from iron sulphate as zinc is more reactive than iron.

Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




