
Raw material for the production of urea are:
A. ammonia and carbon dioxide
B. oxygen and carbon dioxide
C. ammonia and oxygen
D. ammonia and phosphate
Answer
568.8k+ views
Hint: Urea has an Amide functional group. Urea is diamide of carbonic acid. When both hydroxyl groups of carbonic acid are replaced with ammonia groups, the formed structure is known as amide. The structure or constitutes of the product gives the idea about reactants.
Complete step by step solution:The structure of urea is as follows:
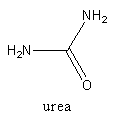
The structure tells that urea contain amine and carbonyl functional groups. The reactant for urea preparation will be ammonia and carbon dioxide. At industrial level, urea is prepared by reacting ammonia with carbon dioxide.
Urea formed at high temperature and pressure. Formation take place in two steps:
First, ammonia and carbon dioxide reacts to give ammonium carbonate. It is a reversible step.
The reaction of formation of ammonia carbonate is as follows:
${\text{N}}{{\text{H}}_{\text{3}}}\,{\text{ + }}\,{\text{C}}{{\text{O}}_{\text{2}}}\, \rightleftarrows \,\,{\text{N}}{{\text{H}}_{\text{2}}}{\text{COON}}{{\text{H}}_{\text{4}}}$
${\text{N}}{{\text{H}}_{\text{2}}}{\text{COON}}{{\text{H}}_{\text{4}}}$ is known as ammonium carbonate.
In the second step, ammonium carbonate decomposes to give urea and water. It is also a reversible step.
${\text{N}}{{\text{H}}_{\text{2}}}{\text{COON}}{{\text{H}}_{\text{4}}}\, \rightleftarrows \,{\text{N}}{{\text{H}}_{\text{2}}}{\text{COON}}{{\text{H}}_{\text{2}}}{\text{ + }}\,{{\text{H}}_{\text{2}}}{\text{O}}\,$
By the same reaction ammonia and carbon dioxide can be recycled. On decreasing pressure and increasing the temperature, ammonium carbonate decomposes into ammonia and carbon dioxide.
So, raw materials for the production of urea are ammonia and carbon dioxide.
Therefore, option (A) ammonia and carbon dioxide, is correct.
Note: Urea has a high amount of nitrogen, so it is used as fertilizer. In the laboratory, urea is prepared by the Wohler method. Urea is also used in manufacturing of resin. Ammonia is the main reactant for the urea production, so urea manufacturing is done nearby ammonia production plants. At industry level, ammonia is produced by the Haber process. In the Haber process, nitrogen is reacted with hydrogen at high temperature in presence of a catalyst to produce ammonia.
Complete step by step solution:The structure of urea is as follows:

The structure tells that urea contain amine and carbonyl functional groups. The reactant for urea preparation will be ammonia and carbon dioxide. At industrial level, urea is prepared by reacting ammonia with carbon dioxide.
Urea formed at high temperature and pressure. Formation take place in two steps:
First, ammonia and carbon dioxide reacts to give ammonium carbonate. It is a reversible step.
The reaction of formation of ammonia carbonate is as follows:
${\text{N}}{{\text{H}}_{\text{3}}}\,{\text{ + }}\,{\text{C}}{{\text{O}}_{\text{2}}}\, \rightleftarrows \,\,{\text{N}}{{\text{H}}_{\text{2}}}{\text{COON}}{{\text{H}}_{\text{4}}}$
${\text{N}}{{\text{H}}_{\text{2}}}{\text{COON}}{{\text{H}}_{\text{4}}}$ is known as ammonium carbonate.
In the second step, ammonium carbonate decomposes to give urea and water. It is also a reversible step.
${\text{N}}{{\text{H}}_{\text{2}}}{\text{COON}}{{\text{H}}_{\text{4}}}\, \rightleftarrows \,{\text{N}}{{\text{H}}_{\text{2}}}{\text{COON}}{{\text{H}}_{\text{2}}}{\text{ + }}\,{{\text{H}}_{\text{2}}}{\text{O}}\,$
By the same reaction ammonia and carbon dioxide can be recycled. On decreasing pressure and increasing the temperature, ammonium carbonate decomposes into ammonia and carbon dioxide.
So, raw materials for the production of urea are ammonia and carbon dioxide.
Therefore, option (A) ammonia and carbon dioxide, is correct.
Note: Urea has a high amount of nitrogen, so it is used as fertilizer. In the laboratory, urea is prepared by the Wohler method. Urea is also used in manufacturing of resin. Ammonia is the main reactant for the urea production, so urea manufacturing is done nearby ammonia production plants. At industry level, ammonia is produced by the Haber process. In the Haber process, nitrogen is reacted with hydrogen at high temperature in presence of a catalyst to produce ammonia.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




