
Rank the following substances in order of decreasing heat of combustion (maximum – minimum):
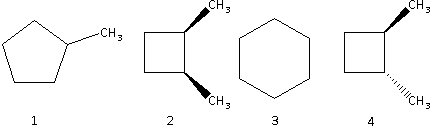
A. $1 > 2 > 3 > 4$
B. $3 > 4 > 2 > 1$
C. $2 > 4 > 1 > 3$
D. $1 > 3 > 2 > 4$

Answer
585.3k+ views
Hint: The heat of combustion is inversely proportional to the stability of the ring. Thus,
${\text{Heat of combustion}} \propto \dfrac{1}{{{\text{Stability of the ring}}}}$.
Complete step by step answer:
Step 1:
Determine the order of stability as follows:
The stability of the ring increases as the size of the ring increases. Substance 2 and substance 4 have the same ring size. The size of substance 1 is greater than substance 2 and 4 and the size of substance 3 is the greatest.
Thus, the order of stability is $3 > 1 > 2 = 4$.
The substances 2 and 4 are isomers. The trans isomer is more stable than the cis isomer. This is because in a trans isomer, the substituent groups are attached on the opposite sides of the carbon – carbon bond which decreases the strain in the ring resulting in an increased stability. Thus, the substance 4 (trans isomer) is more stable than substance 2 (cis isomer).
Thus, the order of stability is $3 > 1 > 4 > 2$.
Step 2:
Determine the order of heat of combustion as follows:
The heat of combustion is inversely proportional to the stability of the ring. Thus,
${\text{Heat of combustion}} \propto \dfrac{1}{{{\text{Stability of the ring}}}}$
Thus, the order of heat of combustion is the opposite of the order of the stability of the ring.
Thus, the order of heat of combustion is $2 > 4 > 1 > 3$.
Thus, the correct option is option (C).
Note: In a trans isomer, the substituent groups are attached on the opposite side of the carbon – carbon bond. In a cis isomer, the substituent groups are attached on the same side of the carbon – carbon bond. As a result, the steric hindrance due to the substituent groups decreases in a trans isomer. Thus, the trans isomer is always more stable than the cis isomer.
${\text{Heat of combustion}} \propto \dfrac{1}{{{\text{Stability of the ring}}}}$.
Complete step by step answer:
Step 1:
Determine the order of stability as follows:
The stability of the ring increases as the size of the ring increases. Substance 2 and substance 4 have the same ring size. The size of substance 1 is greater than substance 2 and 4 and the size of substance 3 is the greatest.
Thus, the order of stability is $3 > 1 > 2 = 4$.
The substances 2 and 4 are isomers. The trans isomer is more stable than the cis isomer. This is because in a trans isomer, the substituent groups are attached on the opposite sides of the carbon – carbon bond which decreases the strain in the ring resulting in an increased stability. Thus, the substance 4 (trans isomer) is more stable than substance 2 (cis isomer).
Thus, the order of stability is $3 > 1 > 4 > 2$.
Step 2:
Determine the order of heat of combustion as follows:
The heat of combustion is inversely proportional to the stability of the ring. Thus,
${\text{Heat of combustion}} \propto \dfrac{1}{{{\text{Stability of the ring}}}}$
Thus, the order of heat of combustion is the opposite of the order of the stability of the ring.
Thus, the order of heat of combustion is $2 > 4 > 1 > 3$.
Thus, the correct option is option (C).
Note: In a trans isomer, the substituent groups are attached on the opposite side of the carbon – carbon bond. In a cis isomer, the substituent groups are attached on the same side of the carbon – carbon bond. As a result, the steric hindrance due to the substituent groups decreases in a trans isomer. Thus, the trans isomer is always more stable than the cis isomer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




