
How many protons do all chlorine atoms have?
Answer
543k+ views
Hint: Number of protons in an element always remains the same and is equal to its atomic number. Also, number of proton = number of electron for the same atom
Complete answer:
It is very well known that chlorine is an element with the symbol Cl and atomic number 17. It belongs to the halogen family and appears between fluorine and bromine in the periodic table. Its properties are mostly intermediate between fluorine(F) and bromine(Br). It is a yellow-green gas at room temperature. Chlorine is an extremely reactive element and a strong oxidizing agent; also it has the third-highest electronegativity on the Pauling scale, behind only oxygen and fluorine.
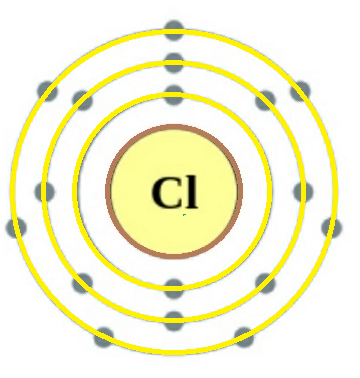
$_{17}^{35.45}Cl$
This is the atomic structure of the Chlorine atom and the way it is denoted is shown beside it. Here we can see that the atomic no. of Cl =17. Also no. of electrons of Cl atom shown in the structure is 17.
As we know that, total no. of proton of an element = atomic no. of the element
Similarly, total no. of electron in an atom = total no. of proton in an atom.
By both ways we can conclude that:-
Total no. of protons in all chlorine atom = 17
Additional Information:
Total no. of neutrons can be calculated as $N=Z-A$
where, A= atomic no. of the element
Z= atomic mass of the element
Note:
Chlorine will always have 17 protons no matter what isotope of chlorine is given, as an element's mass numbers can vary which means that it can have different numbers of neutrons.
From this we conclude that, although chlorine has a mass number of 35 which means it has 18 neutrons and 17 protons, it can also have a mass number of 37, which means it has 20 neutrons and 17 protons.
Complete answer:
It is very well known that chlorine is an element with the symbol Cl and atomic number 17. It belongs to the halogen family and appears between fluorine and bromine in the periodic table. Its properties are mostly intermediate between fluorine(F) and bromine(Br). It is a yellow-green gas at room temperature. Chlorine is an extremely reactive element and a strong oxidizing agent; also it has the third-highest electronegativity on the Pauling scale, behind only oxygen and fluorine.

$_{17}^{35.45}Cl$
This is the atomic structure of the Chlorine atom and the way it is denoted is shown beside it. Here we can see that the atomic no. of Cl =17. Also no. of electrons of Cl atom shown in the structure is 17.
As we know that, total no. of proton of an element = atomic no. of the element
Similarly, total no. of electron in an atom = total no. of proton in an atom.
By both ways we can conclude that:-
Total no. of protons in all chlorine atom = 17
Additional Information:
Total no. of neutrons can be calculated as $N=Z-A$
where, A= atomic no. of the element
Z= atomic mass of the element
Note:
Chlorine will always have 17 protons no matter what isotope of chlorine is given, as an element's mass numbers can vary which means that it can have different numbers of neutrons.
From this we conclude that, although chlorine has a mass number of 35 which means it has 18 neutrons and 17 protons, it can also have a mass number of 37, which means it has 20 neutrons and 17 protons.
Recently Updated Pages
Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Computer Science: Engaging Questions & Answers for Success

Class 10 Question and Answer - Your Ultimate Solutions Guide

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Trending doubts
A boat goes 24 km upstream and 28 km downstream in class 10 maths CBSE

State and explain Ohms law class 10 physics CBSE

Write a letter to the editor of a newspaper explaining class 10 english CBSE

Distinguish between soap and detergent class 10 chemistry CBSE

a Why did Mendel choose pea plants for his experiments class 10 biology CBSE

What is a "free hit" awarded for in limited-overs cricket?




