
Propyl acetate, ${C_5}{H_{10}}{O_2}$ gives the odour and taste to pears. How many moles of $C$ are present in $1.50$ moles of propyl acetate?
Answer
509.7k+ views
Hint: For one mole of a compound, the number of moles of an element in that compound is equivalent to the number of atoms of that element present. For example, in the mole of methane i.e., $C{H_4}$the number of moles of carbon atom and hydrogen atom will be equivalent to $1$ and $4$ moles respectively.
Complete answer:
Propyl acetate is an example of ester and due to its sweet smell and taste, it gives the odour and taste to pears and is also used in fragrances and as additive flavours. The molecular formula of propyl acetate is ${C_5}{H_{10}}{O_2}$ and structurally it is represented as follows:
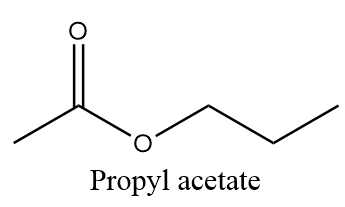
Now, as per question, we need to find out the number of moles of carbon present in $1.50$ moles of propyl acetate. So, the calculation is done in the following way:
Number of moles of carbon in $1$ mole of ${C_5}{H_{10}}{O_2} \Rightarrow 5$moles
Therefore, the number of moles of carbon in $1.50$ moles of ${C_5}{H_{10}}{O_2} \Rightarrow 5 \times 1.50 = 7.5$ moles.
Hence, $7.5$ moles of $C$ are present in $1.50$ moles of propyl acetate.
Note:
Ensure not to get confused between the mass percentage of an element and the number of moles of an element in a compound. The mass percentage of an element in a compound is equal to the ratio of actual amount of that element (i.e., the number of atoms of the element present multiplied by its molar mass) to the molar mass of the compound multiplied by $100$ whereas the number of moles of an atom is equivalent to stoichiometric ratio in which it is present.
Complete answer:
Propyl acetate is an example of ester and due to its sweet smell and taste, it gives the odour and taste to pears and is also used in fragrances and as additive flavours. The molecular formula of propyl acetate is ${C_5}{H_{10}}{O_2}$ and structurally it is represented as follows:

Now, as per question, we need to find out the number of moles of carbon present in $1.50$ moles of propyl acetate. So, the calculation is done in the following way:
Number of moles of carbon in $1$ mole of ${C_5}{H_{10}}{O_2} \Rightarrow 5$moles
Therefore, the number of moles of carbon in $1.50$ moles of ${C_5}{H_{10}}{O_2} \Rightarrow 5 \times 1.50 = 7.5$ moles.
Hence, $7.5$ moles of $C$ are present in $1.50$ moles of propyl acetate.
Note:
Ensure not to get confused between the mass percentage of an element and the number of moles of an element in a compound. The mass percentage of an element in a compound is equal to the ratio of actual amount of that element (i.e., the number of atoms of the element present multiplied by its molar mass) to the molar mass of the compound multiplied by $100$ whereas the number of moles of an atom is equivalent to stoichiometric ratio in which it is present.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




