
What is the property of water that makes it the universal solvent?
Answer
525.9k+ views
Hint: To solve this question we first need to know what is a solvent. A solvent is a substance that forms a solution upon dissolving a solute. Solvent can be gaseous, liquid, solid, or supercritical fluid. Usually, a solvent is a liquid.
Complete answer:
The ability of a substance (or the solute) to dissolve in a liquid (or a solvent) to form a solution is known as solubility.
The structure of water molecules is as follows
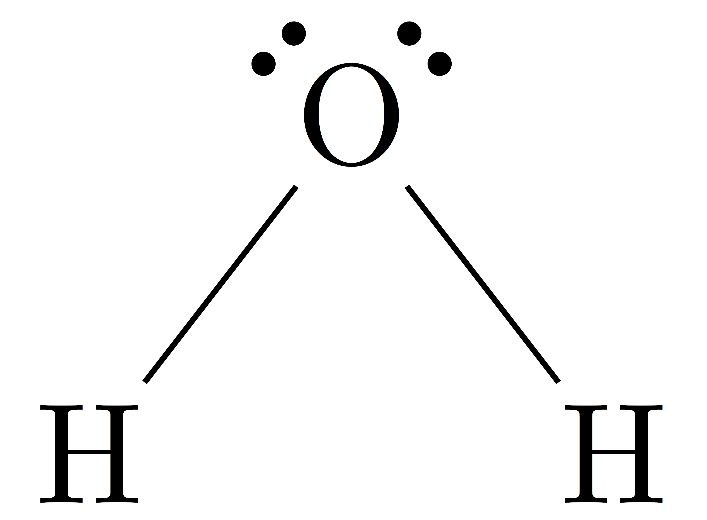
Now, since in water the hydrogen atom is directly attached to an oxygen atom, it forms a polar arrangement as the oxygen atoms form a negative charge and the hydrogen atom forms a positive charge due to the difference in electronegativities of hydrogen and oxygen.
Due to this polarity, water molecules can attract different molecules like sodium chloride (NaCl) and dissolve them by breaking the attractive forces between Na and Cl.
This is why water dissolves a solute more than any other solvent hence it is deemed as a universal solvent.
Water dissolves solutes to form an aqueous solution.
Note:
Water is a protic solvent.
A protic solvent is the one when there is a hydrogen atom bonded to either a
- Nitrogen atom as amine group ($-N{{R}_{3}}$),
- Oxygen atom as hydroxyl group (-OH), or
- Fluoride atom as hydrogen fluoride group (HF)
In these solvents, the hydrogen atom is a liable ${{H}^{+}}$ ion. Solutes can easily gain protons (${{H}^{+}}$) from the molecules of these solvents through hydrogen bonding.
Now, solvents that do not donate ${{H}^{+}}$ or accept protons to the solute are known as polar aprotic solvents. For example, cyclohexane, toluene, etc. They are chemically inert and usually have huge dipole moments and low dielectric constant.
Complete answer:
The ability of a substance (or the solute) to dissolve in a liquid (or a solvent) to form a solution is known as solubility.
The structure of water molecules is as follows

Now, since in water the hydrogen atom is directly attached to an oxygen atom, it forms a polar arrangement as the oxygen atoms form a negative charge and the hydrogen atom forms a positive charge due to the difference in electronegativities of hydrogen and oxygen.
Due to this polarity, water molecules can attract different molecules like sodium chloride (NaCl) and dissolve them by breaking the attractive forces between Na and Cl.
This is why water dissolves a solute more than any other solvent hence it is deemed as a universal solvent.
Water dissolves solutes to form an aqueous solution.
Note:
Water is a protic solvent.
A protic solvent is the one when there is a hydrogen atom bonded to either a
- Nitrogen atom as amine group ($-N{{R}_{3}}$),
- Oxygen atom as hydroxyl group (-OH), or
- Fluoride atom as hydrogen fluoride group (HF)
In these solvents, the hydrogen atom is a liable ${{H}^{+}}$ ion. Solutes can easily gain protons (${{H}^{+}}$) from the molecules of these solvents through hydrogen bonding.
Now, solvents that do not donate ${{H}^{+}}$ or accept protons to the solute are known as polar aprotic solvents. For example, cyclohexane, toluene, etc. They are chemically inert and usually have huge dipole moments and low dielectric constant.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




