
What property of hydrogen chloride is demonstrated by this experiment?
(A) Acidic
(B) Oxidising nature
(C) Dehydrating
(D) None of these

Answer
584.7k+ views
Hint: It is known that the hydrochloric acid is a very strong acid having a large value of dissociation or ionization constant and it dissociates completely in water. Acid causes the change of blue litmus paper to red.
Complete step by step solution:
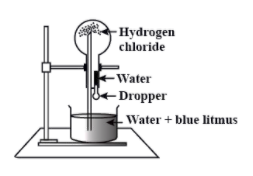
-In the given diagram, it is seen that the round bottom flask has hydrogen chloride gas. A glass tube inserted into the flask is placed inverted into the beaker containing water and blue litmus indicator. The flask is also sealed and has a dropper filled with water. A fountain is produced in the flask. This is well known as the Fountain experiment.
-In this experiment, it is initiated by injecting water from the dropper into the flask. The hydrogen chloride gas present in the flask dissolves in presence of water creating a partial vacuum. Thereby, the air pressure around the tube causes the water level to rise and squeezes the water into the flask in the form of a fountain.
-It is found that the fountain produced is red in colour. Therefore, the change in colour of the blue litmus solution into the red colour fountain shows the acidic nature of hydrogen chloride gas used in the experiment.
\[HCl\text{ }\left( g \right)\text{ +}{{\text{H}}_{2}}\text{O}\to \text{ }{{H}_{3}}{{O}^{+}}\left( aq \right)\text{ }+\text{ }C{{l}^{-}}\left( aq \right)\]
-Thus, the hydrogen chloride gas being highly soluble in water gives hydrochloric acid. This hydrochloric acid is what causes the change in colour of the blue litmus solution to red colour, and contributes in its acidic property.
Hence, the correct answer is option is (A).
Note: This experiment is also used in order to study the properties of ammonia and sulphur dioxide gas as these gases are also highly soluble in water producing the acidic solution.
Complete step by step solution:
-In the given diagram, it is seen that the round bottom flask has hydrogen chloride gas. A glass tube inserted into the flask is placed inverted into the beaker containing water and blue litmus indicator. The flask is also sealed and has a dropper filled with water. A fountain is produced in the flask. This is well known as the Fountain experiment.
-In this experiment, it is initiated by injecting water from the dropper into the flask. The hydrogen chloride gas present in the flask dissolves in presence of water creating a partial vacuum. Thereby, the air pressure around the tube causes the water level to rise and squeezes the water into the flask in the form of a fountain.
-It is found that the fountain produced is red in colour. Therefore, the change in colour of the blue litmus solution into the red colour fountain shows the acidic nature of hydrogen chloride gas used in the experiment.
\[HCl\text{ }\left( g \right)\text{ +}{{\text{H}}_{2}}\text{O}\to \text{ }{{H}_{3}}{{O}^{+}}\left( aq \right)\text{ }+\text{ }C{{l}^{-}}\left( aq \right)\]
-Thus, the hydrogen chloride gas being highly soluble in water gives hydrochloric acid. This hydrochloric acid is what causes the change in colour of the blue litmus solution to red colour, and contributes in its acidic property.
Hence, the correct answer is option is (A).
Note: This experiment is also used in order to study the properties of ammonia and sulphur dioxide gas as these gases are also highly soluble in water producing the acidic solution.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




