
Propene \[C{{H}_{3}}-CH=C{{H}_{2}}\]can be converted into 1-propanol by oxidation.
Indicate which sets of reagents amongst the following is ideal to effect the above conversion?
(a)- \[KMn{{O}_{4}}\] (alkaline)
(b)- Osmium tetroxide (\[Os{{O}_{4}}/C{{H}_{2}}C{{l}_{2}}\])
(c)- \[{{B}_{2}}{{H}_{6}}\] and alk \[{{H}_{2}}{{O}_{2}}\]
(d)- \[{{O}_{3}}\] and Zn/\[{{H}_{2}}O\]
Answer
597.9k+ views
Hint: Herbert C. Brown developed the hydroboration technique, mostly carried out at Purdue University and won the Nobel Prize in 1979 for his invention. It paved a new way to practice organic chemistry.
Complete step by step solution:
Hydroboration-oxidation reaction is used to convert alkene into alcohol. In this reaction, borane from the borane compound gets added across the carbon‐carbon double bond of the alkene. In other words, the addition of borane will happen at the double bond in such a manner that the boron atom gets attached to the $sp^2$ carbon carrying a greater number of hydrogen atoms. As a result, an organoboron compound (trialkyl borane) is formed.
Again, this formed organoboron compound reacts with hydrogen peroxide in a basic medium and oxidizes to alcohol by the addition of water to the alkene in a way opposite to Markovnikov's rule. This results in regiochemistry of this reaction following anti-Markovnikov’s rule, i.e., hydrogen goes to the more substituted carbon atom of the alkene.
Stereochemistry of this reaction is syn, i.e. boron and hydrogen are on the same side of the product.
The reaction can be given as:
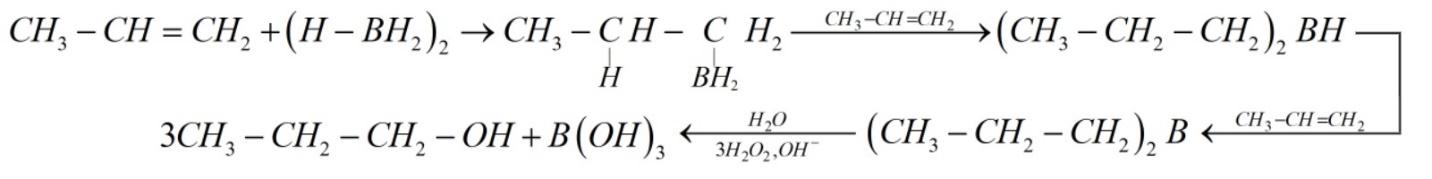
So, the correct option is (c).
Note: The boron byproduct formed in the reaction depends on the number of equivalents of the boron compound used relative to the alkene. The reaction will be the same if \[B{{H}_{3}}\] is used. \[B{{H}_{3}}\]-THF is the same as \[B{{H}_{3}}\]. Tetrahydrofuran (THF) is merely a solvent. \[{{B}_{2}}{{H}_{6}}\] is another form of \[B{{H}_{3}}\]. It behaves the same way as \[B{{H}_{3}}\].
Complete step by step solution:
Hydroboration-oxidation reaction is used to convert alkene into alcohol. In this reaction, borane from the borane compound gets added across the carbon‐carbon double bond of the alkene. In other words, the addition of borane will happen at the double bond in such a manner that the boron atom gets attached to the $sp^2$ carbon carrying a greater number of hydrogen atoms. As a result, an organoboron compound (trialkyl borane) is formed.
Again, this formed organoboron compound reacts with hydrogen peroxide in a basic medium and oxidizes to alcohol by the addition of water to the alkene in a way opposite to Markovnikov's rule. This results in regiochemistry of this reaction following anti-Markovnikov’s rule, i.e., hydrogen goes to the more substituted carbon atom of the alkene.
Stereochemistry of this reaction is syn, i.e. boron and hydrogen are on the same side of the product.
The reaction can be given as:
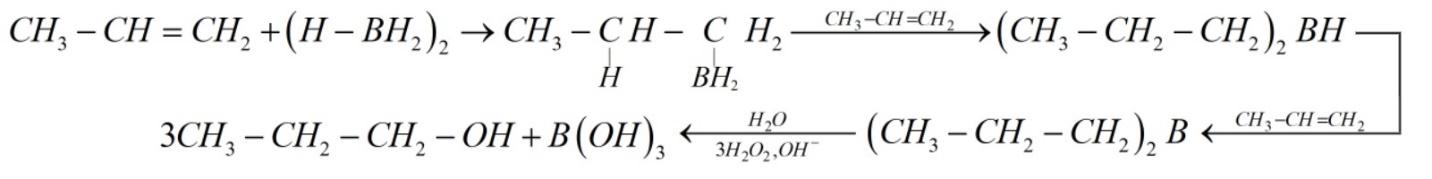
So, the correct option is (c).
Note: The boron byproduct formed in the reaction depends on the number of equivalents of the boron compound used relative to the alkene. The reaction will be the same if \[B{{H}_{3}}\] is used. \[B{{H}_{3}}\]-THF is the same as \[B{{H}_{3}}\]. Tetrahydrofuran (THF) is merely a solvent. \[{{B}_{2}}{{H}_{6}}\] is another form of \[B{{H}_{3}}\]. It behaves the same way as \[B{{H}_{3}}\].
Recently Updated Pages
Master Class 12 Business Studies: Engaging Questions & Answers for Success

Master Class 12 Biology: Engaging Questions & Answers for Success

Master Class 12 Chemistry: Engaging Questions & Answers for Success

Class 12 Question and Answer - Your Ultimate Solutions Guide

Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




