
How many products will be formed excluding stereo when cis-1,3,5-trimethyl cyclohexane reacts with NBS?
(A) 3
(B) 4
(C) 5
(D) 6
Answer
577.5k+ views
Hint: NBS stands for N-bromo succinimide. When it is allowed to react with alkenes, it does allylic bromination. Allylic bromination is a type of substitution reaction where hydrogen atom gets substituted by bromine atom.
Complete answer:
First of all, we will get information about NBS and then see the structure of the substrate in order to find the number of products formed.
- NBS stands for N-bromo succinimide. It is known for allylic bromination. Thus, it substitutes –Br atom in place of –H atom and gives substitution reaction.
- The structure of the substrate cis-1,3,5-trimethyl cyclohexane can be given as
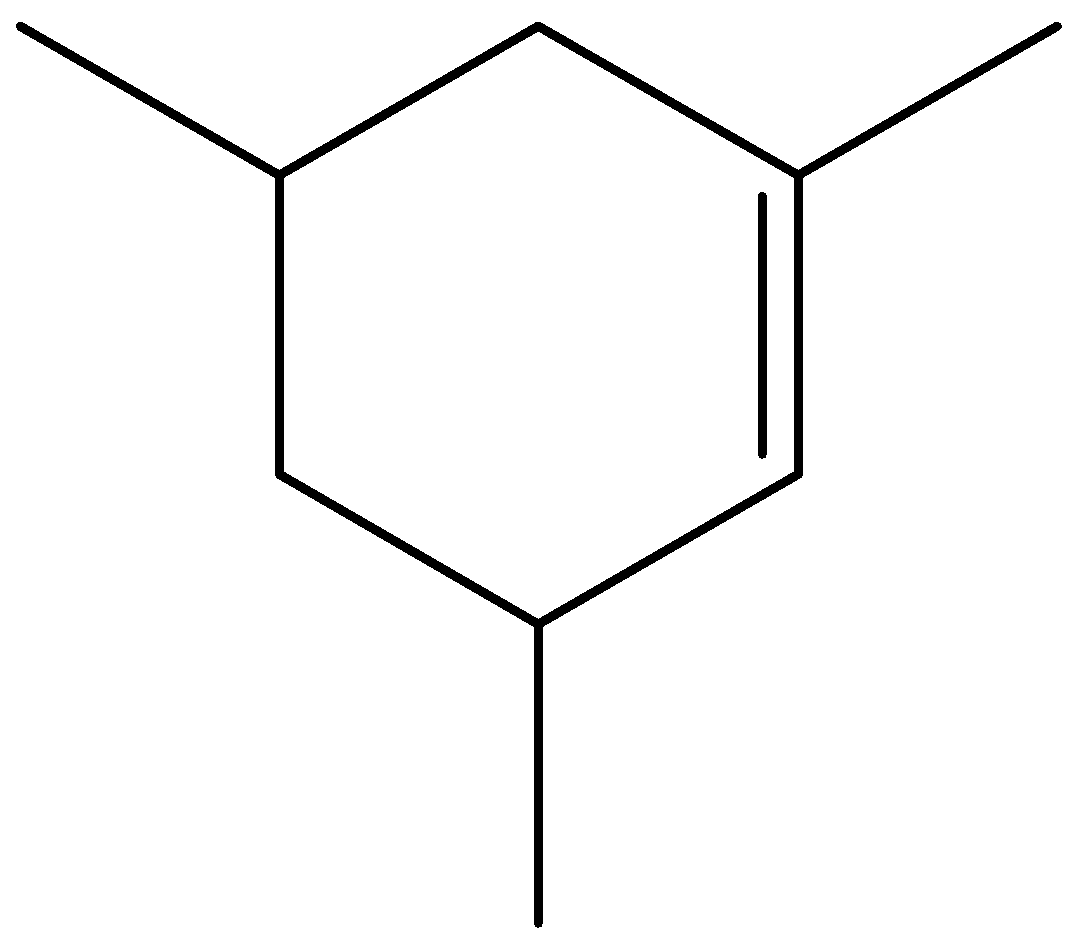
- We know that the hydrogen atom at allylic position can be substituted by bromine atom by the reagent NBS. Allylic carbon is the carbon which is bonded with a single bond to the $s{p^2}$ hybridized carbon.
- We can see that there are three allylic carbons in the compound. All the allylic carbons have at least one hydrogen atom. So, they can give this reaction. So, we can say that a total of 3 products will be formed in this reaction given as below.
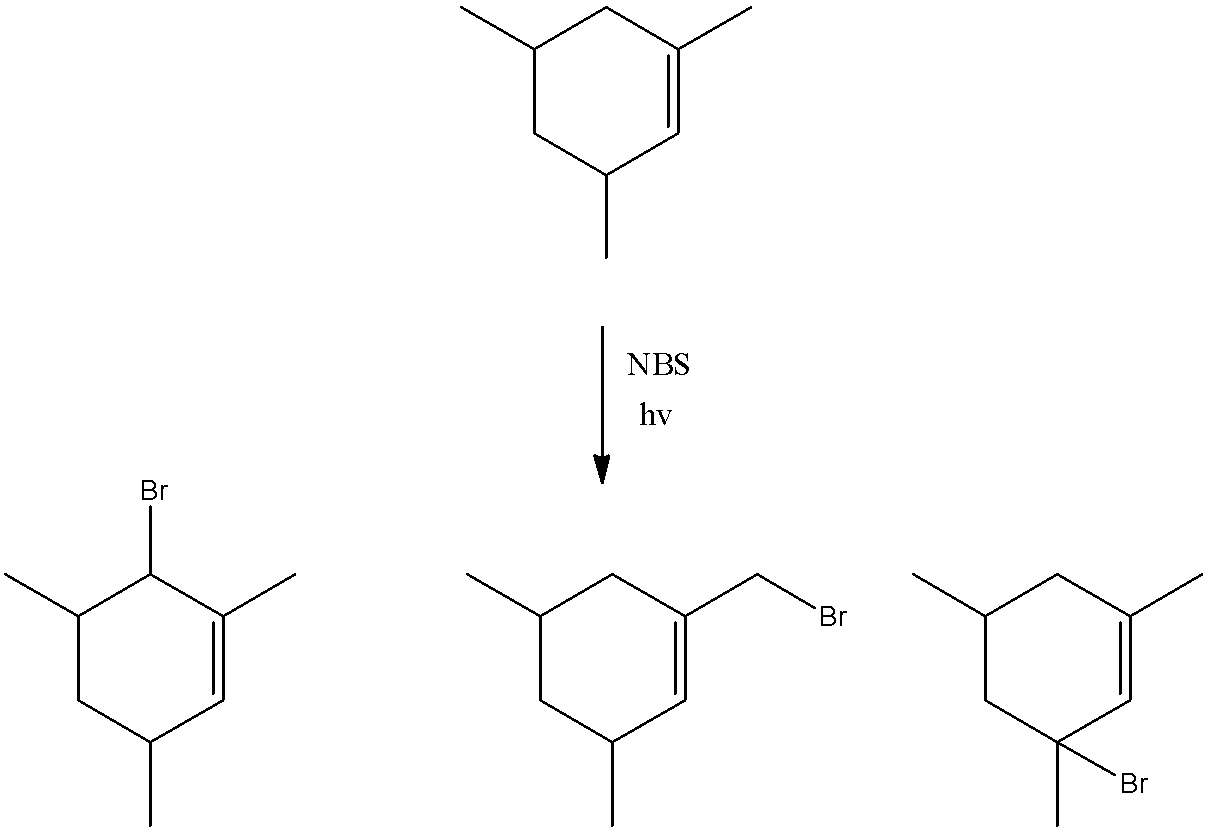
- We can see that light is used in this reaction. This reaction proceeds via a free radical mechanism. The substitution of bromine atoms occurs at allylic position because allylic free radical is very stable.
Thus, we can conclude that a total of three products will be formed in the given reaction.
So, the correct answer of the question is (A).
Note:
In the presence of acids, NBS can also do $\alpha $-bromination to carbonyl derivatives. In presence of water and THF as solvent, NBS can give additional reactions as well where –OH and –Br groups are added across the double bond.
Complete answer:
First of all, we will get information about NBS and then see the structure of the substrate in order to find the number of products formed.
- NBS stands for N-bromo succinimide. It is known for allylic bromination. Thus, it substitutes –Br atom in place of –H atom and gives substitution reaction.
- The structure of the substrate cis-1,3,5-trimethyl cyclohexane can be given as

- We know that the hydrogen atom at allylic position can be substituted by bromine atom by the reagent NBS. Allylic carbon is the carbon which is bonded with a single bond to the $s{p^2}$ hybridized carbon.
- We can see that there are three allylic carbons in the compound. All the allylic carbons have at least one hydrogen atom. So, they can give this reaction. So, we can say that a total of 3 products will be formed in this reaction given as below.

- We can see that light is used in this reaction. This reaction proceeds via a free radical mechanism. The substitution of bromine atoms occurs at allylic position because allylic free radical is very stable.
Thus, we can conclude that a total of three products will be formed in the given reaction.
So, the correct answer of the question is (A).
Note:
In the presence of acids, NBS can also do $\alpha $-bromination to carbonyl derivatives. In presence of water and THF as solvent, NBS can give additional reactions as well where –OH and –Br groups are added across the double bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




