
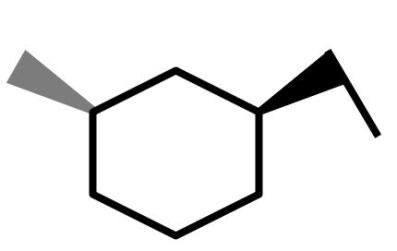
What is the product of the following reactions?
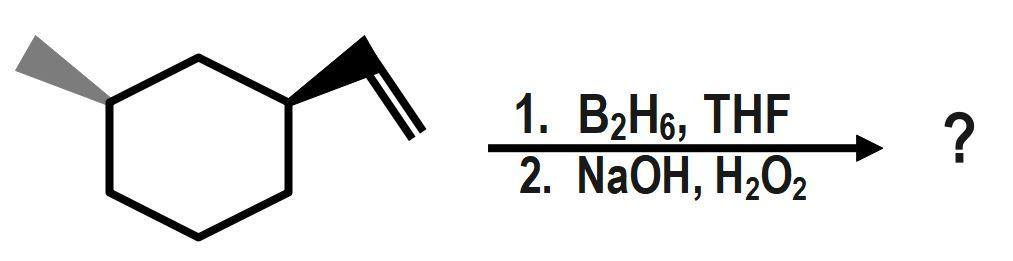
(A)
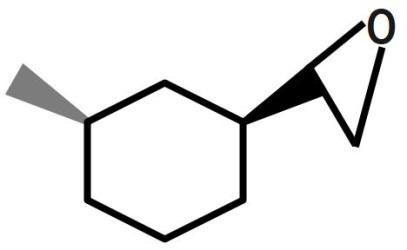
(B)
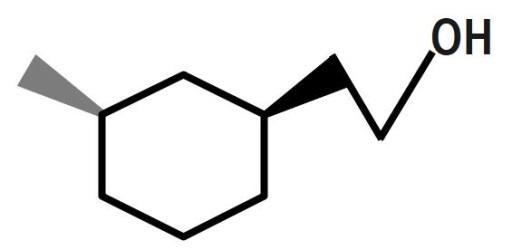
(C)
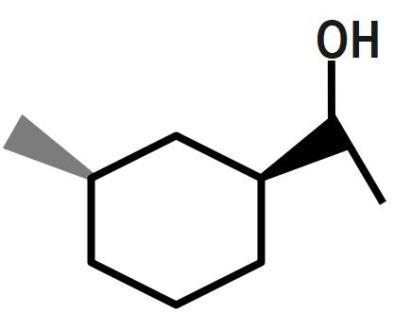
(D)
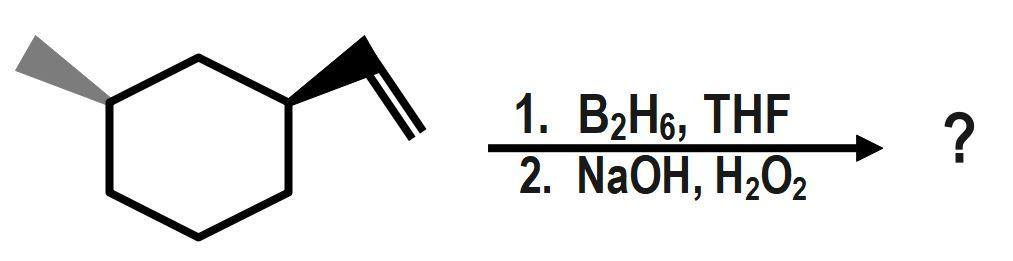




Answer
512.1k+ views
Hint :We know that the hydroboration oxidation is an anti-markovnikov addition of water across alkene. Also, it is a two-step organic reaction. Hydroboration oxidation reaction is another important chemical reaction of organic chemistry which converts alkene into an alcohol. This reaction gives a more stereo specific and regiochemical alternative to other hydration reactions such as acid-catalyzed addition and oxymercuration reduction reactions.
Complete Step By Step Answer:
Hydroboration oxidation reaction is a two-step reaction in which alkene reacts with $ B{{H}_{3}}, $ THF (tetrahydrofuran) and hydrogen peroxide in a basic medium to give alcohol. It is also known as $ HBO $ reaction. Hydroboration oxidation of symmetrical alkene and unsymmetrical alkene works differently. As the reaction proceeds with unsymmetrical alkene follows Anti-Markovnikov rule. Although as a product we always get alcohol from both alkenes.
It is known to you that hydroboration oxidation is a two-step organic reaction. It is an organic reaction that converts an alkene into an alcohol by the net addition of water across the double bond. THF (Tetrahydrofuran) is used as a solvent. The hydrogen $ \left( H \right) $ and hydroxyl group $ \left( OH \right) $ are added in a syn (addition of addendum on the same side of the alkene or alkyne) addition leading to cis stereochemistry. Hydroboration-oxidation is an anti-Markovnikov’s addition of water across an alkene. The $ -OH $ group is added to the less substituted position and the -H is in the more substituted position. If there is more than one chiral center in the product, then the product will produce a pair of enantiomers that results from syn addition.
Here, we can see that first, is added to the carbon-carbon double bond in a way that $ H- $ atoms get bonded to a carbon that has less number of hydrogen atoms(means in an anti-markovnikov manner). Then, in presence of hydrogen peroxide-an oxidizing agent, the organo-borane compound decomposes and gives an alcohol as shown in the reaction. One molecule can oxidise three molecules of alkene because one $ B-H $ bond is responsible for the oxidation of one molecule of alkene.
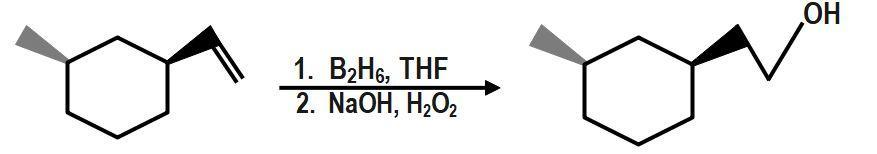
Therefore, the correct answer is option B.
Note :
Remember that it's necessary to understand the chemistry of $ B{{H}_{3}} $ before understanding the mechanism of hydroboration oxidation reaction. As boron has one p-orbital empty so $ B{{H}_{3}} $ acts as Lewis acid. It exists as a dimer of $ B{{H}_{3}}, $ it means it exists in the form of $ {{B}_{2}}{{H}_{6}}2B{{H}_{3}}\to {{B}_{2}}{{H}_{6}} $ .
Complete Step By Step Answer:
Hydroboration oxidation reaction is a two-step reaction in which alkene reacts with $ B{{H}_{3}}, $ THF (tetrahydrofuran) and hydrogen peroxide in a basic medium to give alcohol. It is also known as $ HBO $ reaction. Hydroboration oxidation of symmetrical alkene and unsymmetrical alkene works differently. As the reaction proceeds with unsymmetrical alkene follows Anti-Markovnikov rule. Although as a product we always get alcohol from both alkenes.
It is known to you that hydroboration oxidation is a two-step organic reaction. It is an organic reaction that converts an alkene into an alcohol by the net addition of water across the double bond. THF (Tetrahydrofuran) is used as a solvent. The hydrogen $ \left( H \right) $ and hydroxyl group $ \left( OH \right) $ are added in a syn (addition of addendum on the same side of the alkene or alkyne) addition leading to cis stereochemistry. Hydroboration-oxidation is an anti-Markovnikov’s addition of water across an alkene. The $ -OH $ group is added to the less substituted position and the -H is in the more substituted position. If there is more than one chiral center in the product, then the product will produce a pair of enantiomers that results from syn addition.
Here, we can see that first, is added to the carbon-carbon double bond in a way that $ H- $ atoms get bonded to a carbon that has less number of hydrogen atoms(means in an anti-markovnikov manner). Then, in presence of hydrogen peroxide-an oxidizing agent, the organo-borane compound decomposes and gives an alcohol as shown in the reaction. One molecule can oxidise three molecules of alkene because one $ B-H $ bond is responsible for the oxidation of one molecule of alkene.

Therefore, the correct answer is option B.
Note :
Remember that it's necessary to understand the chemistry of $ B{{H}_{3}} $ before understanding the mechanism of hydroboration oxidation reaction. As boron has one p-orbital empty so $ B{{H}_{3}} $ acts as Lewis acid. It exists as a dimer of $ B{{H}_{3}}, $ it means it exists in the form of $ {{B}_{2}}{{H}_{6}}2B{{H}_{3}}\to {{B}_{2}}{{H}_{6}} $ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




