
what is the product of the following reaction?
$ C{H_3}CHO\xrightarrow[\vartriangle ]{{Se{O_2}}} $
Answer
507.3k+ views
Hint: Selenium dioxide is an oxidizing agent. Selenium dioxide oxidizes allylic positions of alkenes to either alcohols or carbonyl groups. It also oxidizes alpha methylene group on a carbonyl compound to give 1, 2 dicarbonyl compounds. Selenium dioxide is an acidic oxide and dissolves in water to form selenous acid with molecular formula $ {H_2}Se{O_3} $ . Moreover, selenium dioxide exists as a one dimensional polymeric chain with alternating selenium and oxygen atoms.
Complete answer:
The Riley oxidation is a type of selenium dioxide mediated oxidation of alpha methylene groups adjacent to carbonyls. The reaction of acetaldehyde with selenium dioxide involves the following steps:
Step 1: acetaldehyde undergo keto-enol tautomerism:

During the keto-enol tautomerism of acetaldehyde deprotonation of alpha carbon atoms takes place leading to the formation of enolate ions, this is followed by protonation of the enolate ion to form an enol.
Step 2: Selenium dioxide attacks the enol form:
Selenium dioxide attacks the enol from the product as shown.
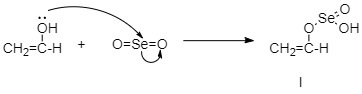
Step 3: Oxidative rearrangement:
The above product undergoes oxidative rearrangement.
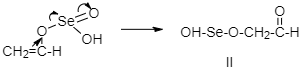
Step 4: Removal of water and selenium:
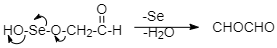
There is a removal of selenium and water from the above product to form the final product which is glyoxal.
Note:
The smallest aldehyde with the chemical formula $ OCHCHO $ is glyoxal. Glyoxal is a crystalline solid which appears to be white at low temperature and yellow near the melting point. In liquid state it appears yellow while in vapour form it is green in color. At commercial or large scale it can be prepared either by gas phase oxidation of ethylene glycol in the presence of a catalyst such as copper or silver or by liquid phase oxidation of acetaldehyde with nitric acid.
Complete answer:
The Riley oxidation is a type of selenium dioxide mediated oxidation of alpha methylene groups adjacent to carbonyls. The reaction of acetaldehyde with selenium dioxide involves the following steps:
Step 1: acetaldehyde undergo keto-enol tautomerism:

During the keto-enol tautomerism of acetaldehyde deprotonation of alpha carbon atoms takes place leading to the formation of enolate ions, this is followed by protonation of the enolate ion to form an enol.
Step 2: Selenium dioxide attacks the enol form:
Selenium dioxide attacks the enol from the product as shown.
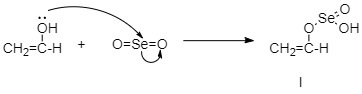
Step 3: Oxidative rearrangement:
The above product undergoes oxidative rearrangement.

Step 4: Removal of water and selenium:
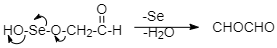
There is a removal of selenium and water from the above product to form the final product which is glyoxal.
Note:
The smallest aldehyde with the chemical formula $ OCHCHO $ is glyoxal. Glyoxal is a crystalline solid which appears to be white at low temperature and yellow near the melting point. In liquid state it appears yellow while in vapour form it is green in color. At commercial or large scale it can be prepared either by gas phase oxidation of ethylene glycol in the presence of a catalyst such as copper or silver or by liquid phase oxidation of acetaldehyde with nitric acid.
Recently Updated Pages
Master Class 12 Business Studies: Engaging Questions & Answers for Success

Master Class 12 Biology: Engaging Questions & Answers for Success

Master Class 12 Chemistry: Engaging Questions & Answers for Success

Class 12 Question and Answer - Your Ultimate Solutions Guide

Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE




