
What is the product of the following reaction?
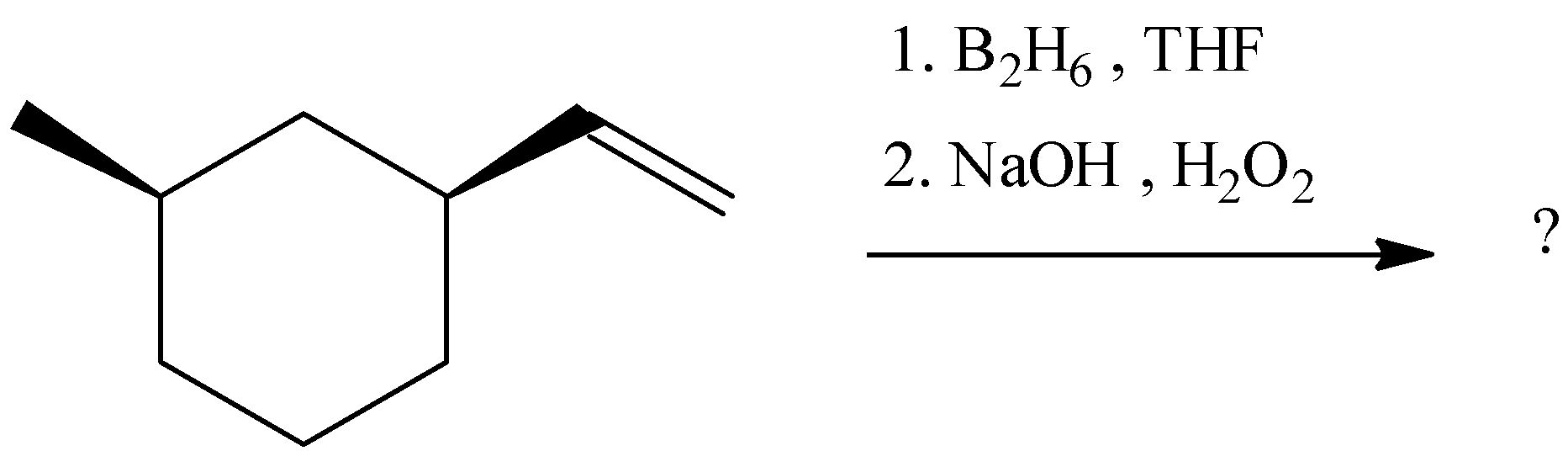
(A)
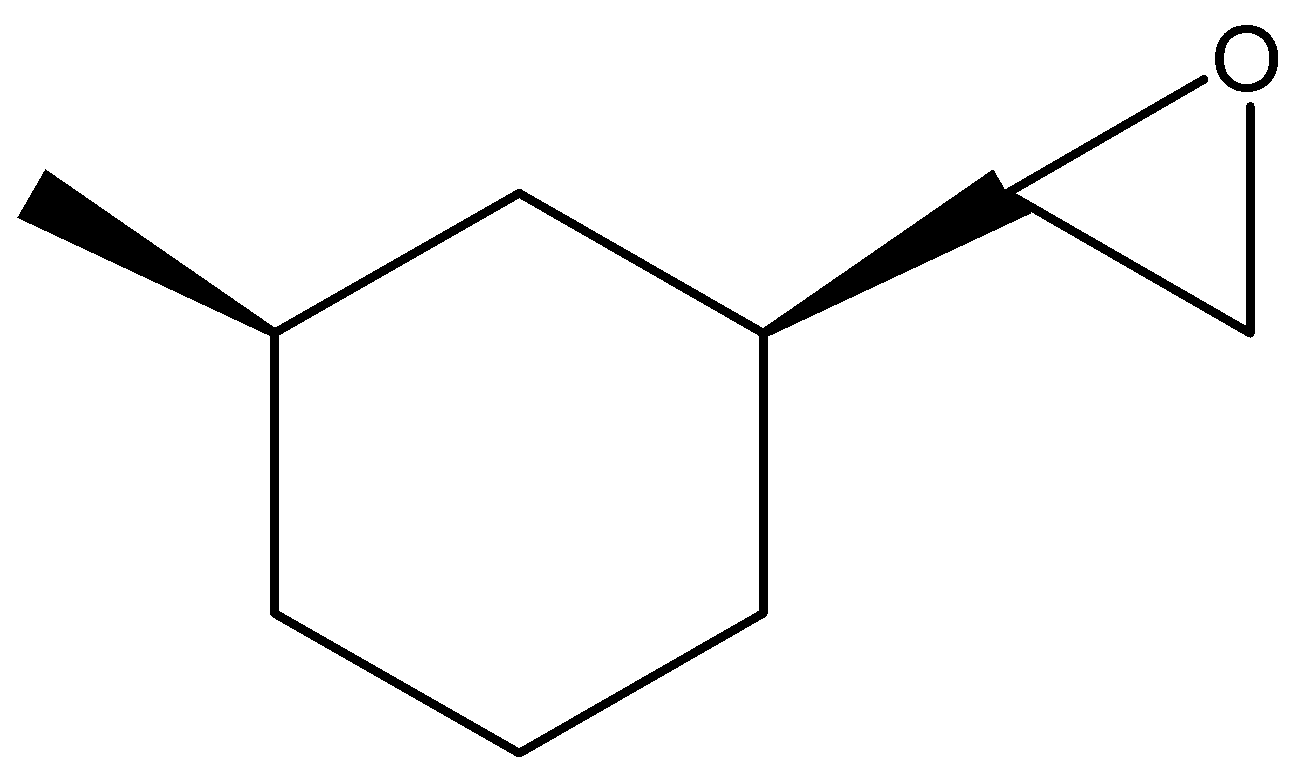
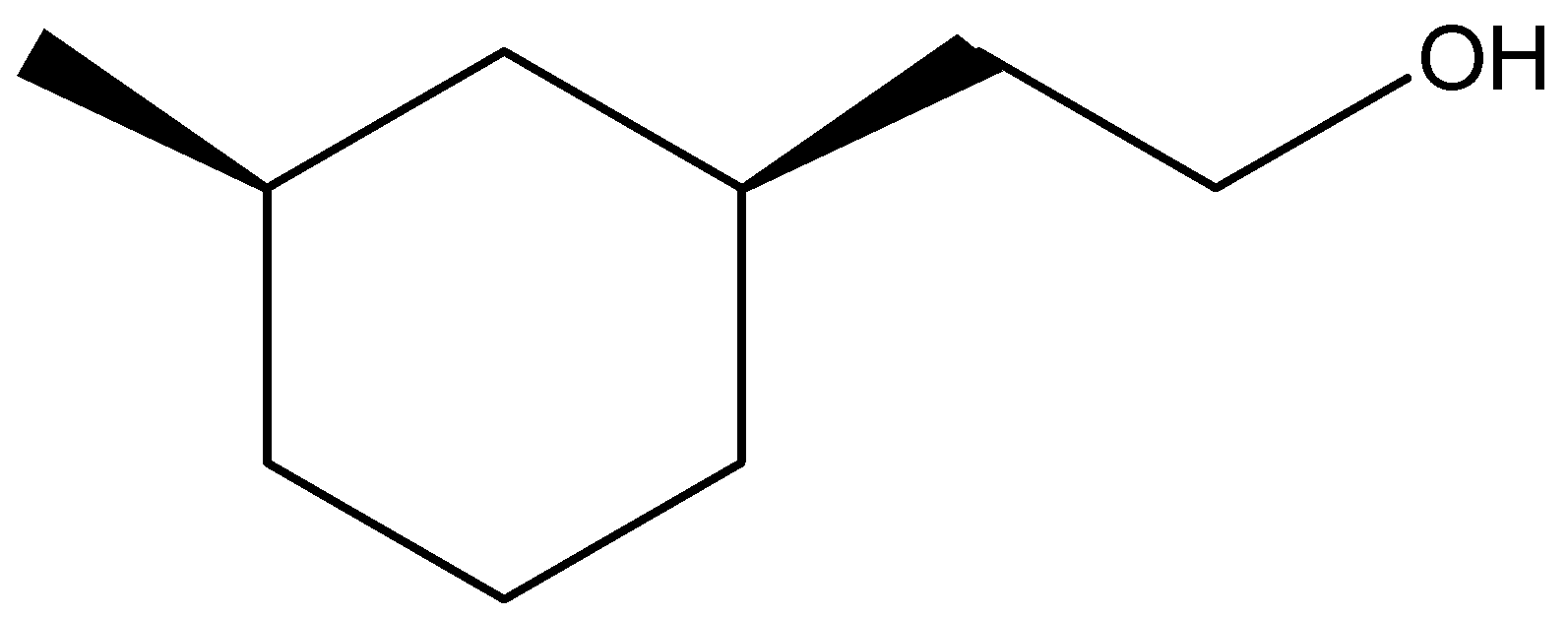
(B)
(C)
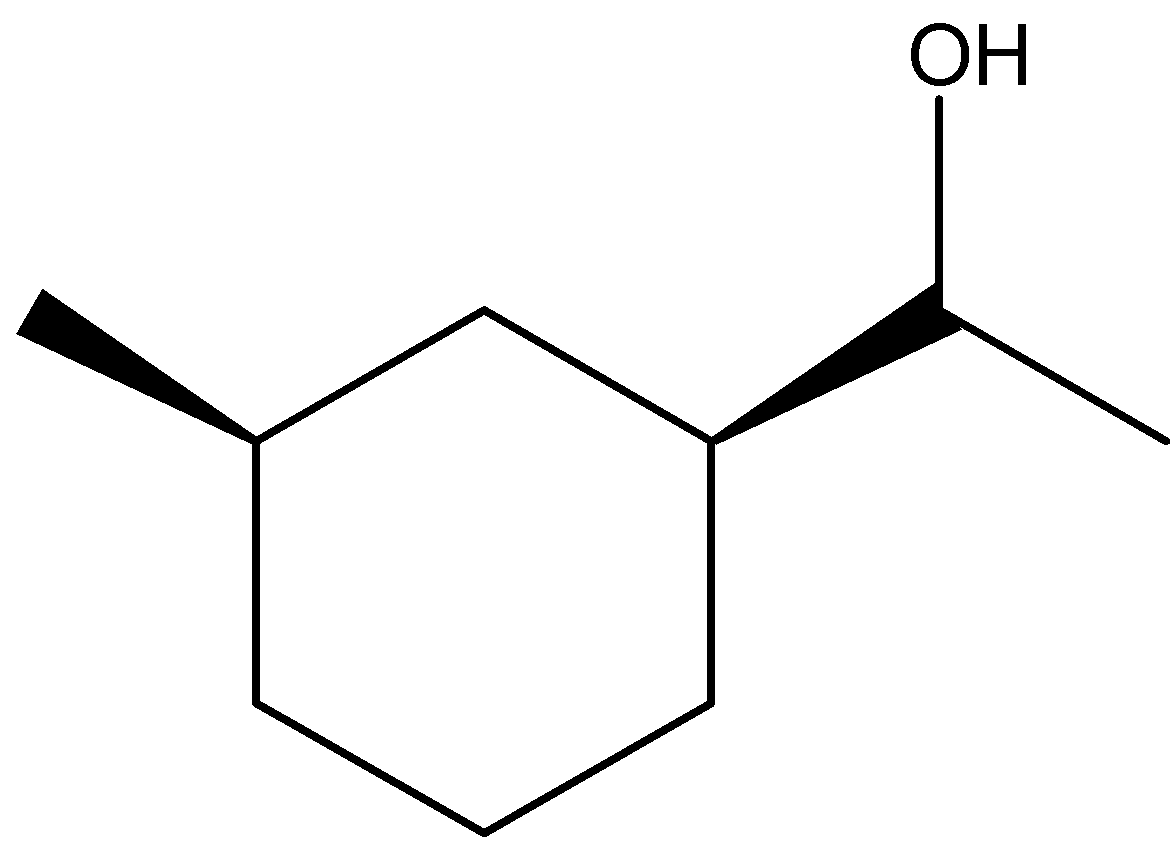
(D)
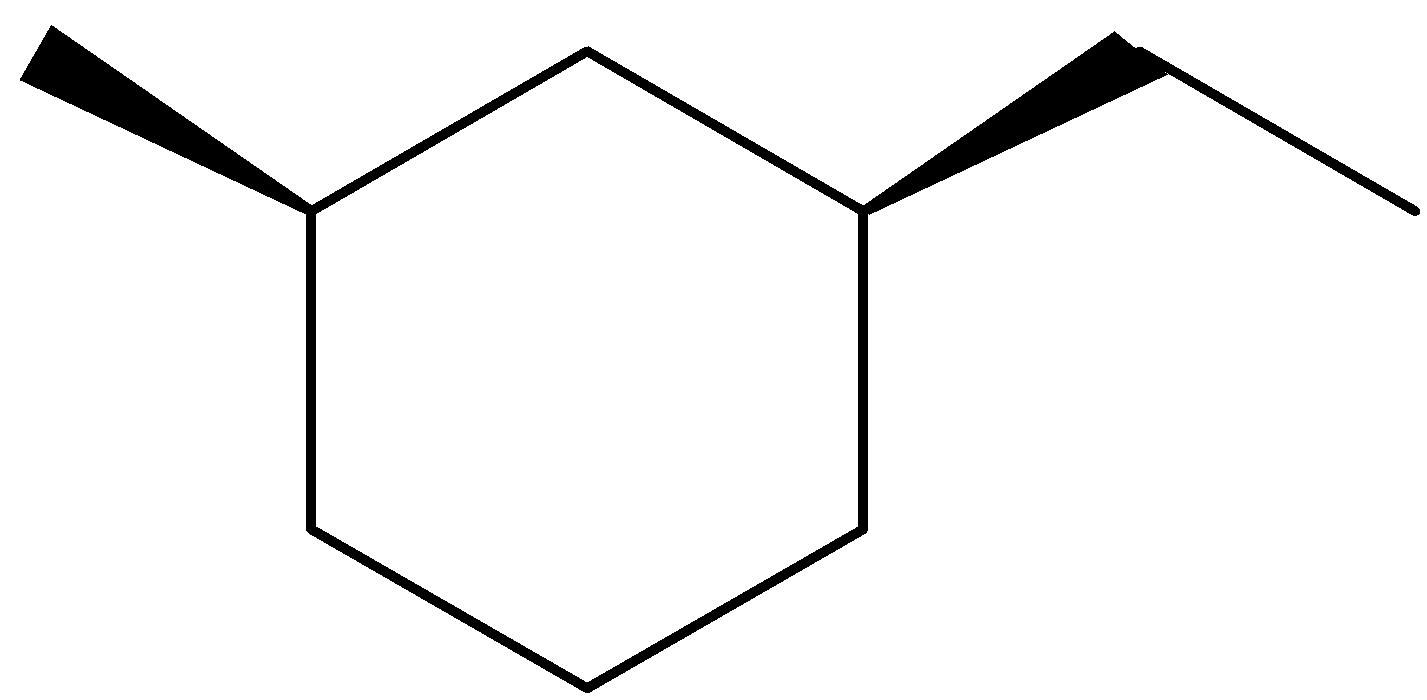





Answer
578.1k+ views
Hint: This is a hydroboration-oxidation reaction. In the first step, a hydroboration reaction will occur across the carbon-carbon double bond. Then, peroxide ions will oxidize the boron and the organic compound.
Complete answer:
We will see the reaction step by step with mechanism.
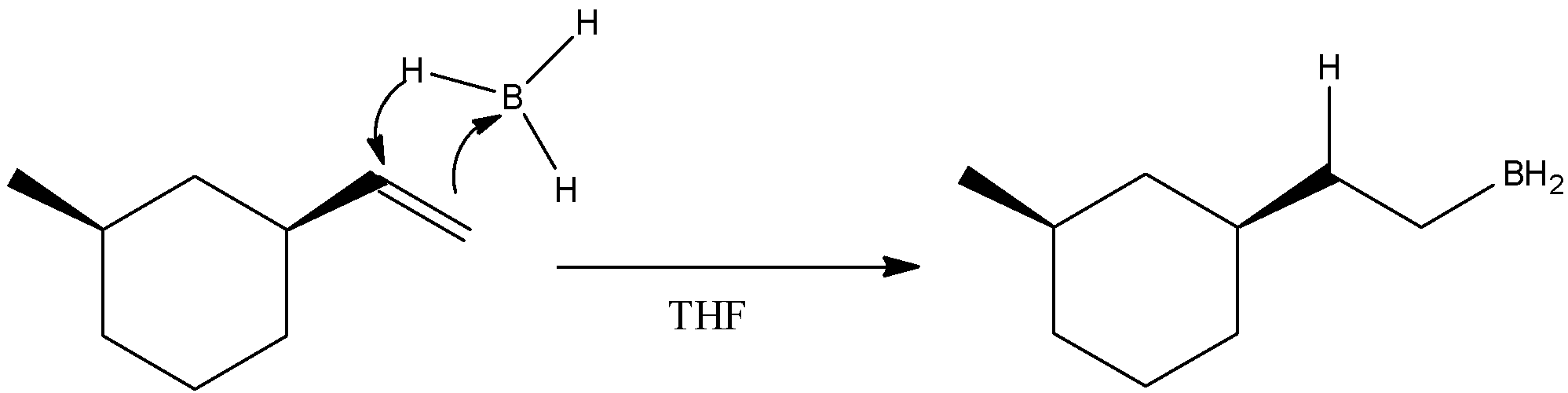
- ${B_2}{H_6}$ is known as diborane and in solvent THF ( Tetrahydrofuran), it undergoes hydroboration reaction when allowed to react with an alkene. So, our starting material is also an alkene. So, a hydroboration reaction will occur in which double bonds between the carbon atoms in alkene will get broken and one carbon will form a bond with hydrogen atom while the other carbon atom will form a bond with $ - B{H_2}$. We know that diborane is a dimer of borane. The mechanism of the first step can be given as
- Then in the second step, the product is allowed to react with NaOH and hydrogen peroxide. The combination of both these reagents is an oxidizing mixture. So, an oxidation reaction will occur here. ${H_2}{O_2}$ on reaction with NaOH gives peroxide ions.
\[{H_2}{O_2} + O{H^ - } \to {H_2}O + HO{O^ - }\]
- These peroxide ions are nucleophilic and attack on the electrophilic boron atom of the organo-boron compound. The reaction is shown with mechanism as below.
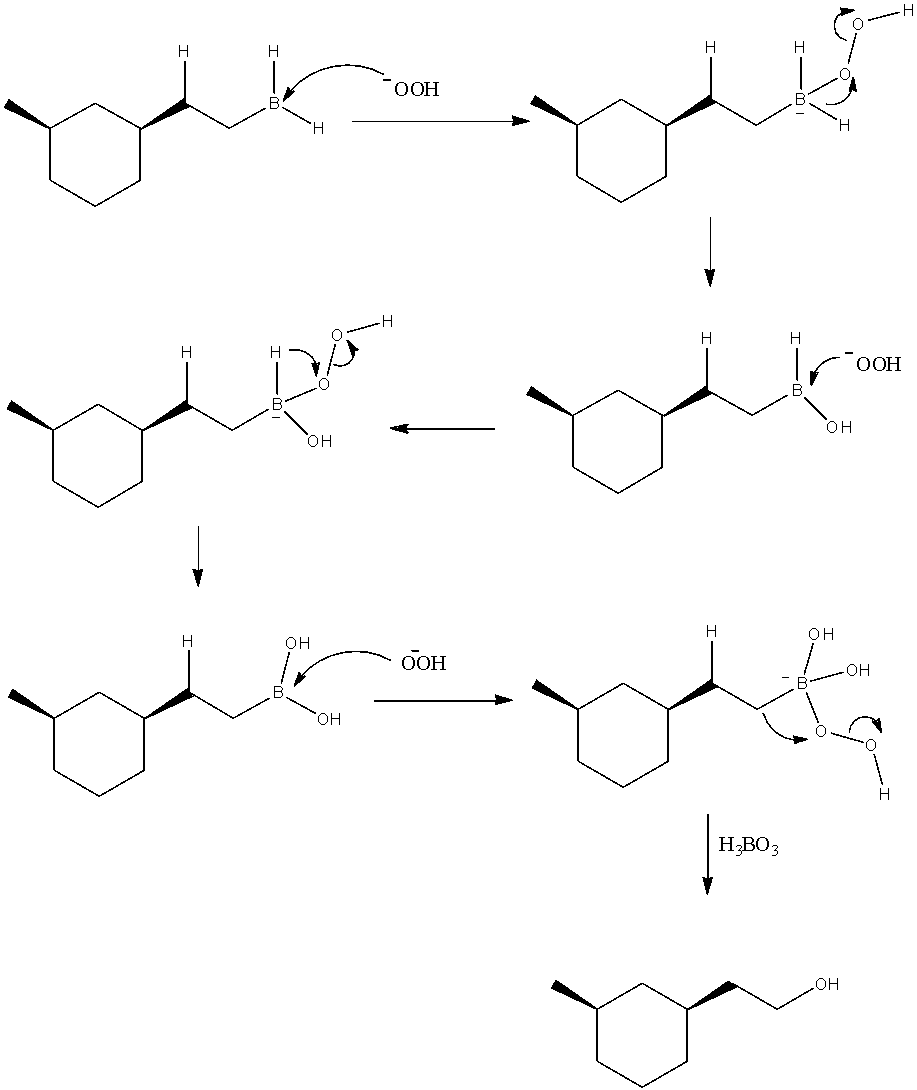
Thus, we can see that upon three cycles of attack of peroxide ions, finally we obtain ${H_3}B{O_3}$ and the corresponding alcohol of the organic compound.
Thus, we can conclude that the answer of the question is (B).
Note:
Note that in hydroboration reaction, the hydrogen atom and hydroxyl group in the final product is syn to each other. Thus, this is syn-addition reaction. The product is the same as the anti-markovnikov addition product.
Complete answer:
We will see the reaction step by step with mechanism.
- ${B_2}{H_6}$ is known as diborane and in solvent THF ( Tetrahydrofuran), it undergoes hydroboration reaction when allowed to react with an alkene. So, our starting material is also an alkene. So, a hydroboration reaction will occur in which double bonds between the carbon atoms in alkene will get broken and one carbon will form a bond with hydrogen atom while the other carbon atom will form a bond with $ - B{H_2}$. We know that diborane is a dimer of borane. The mechanism of the first step can be given as

- Then in the second step, the product is allowed to react with NaOH and hydrogen peroxide. The combination of both these reagents is an oxidizing mixture. So, an oxidation reaction will occur here. ${H_2}{O_2}$ on reaction with NaOH gives peroxide ions.
\[{H_2}{O_2} + O{H^ - } \to {H_2}O + HO{O^ - }\]
- These peroxide ions are nucleophilic and attack on the electrophilic boron atom of the organo-boron compound. The reaction is shown with mechanism as below.

Thus, we can see that upon three cycles of attack of peroxide ions, finally we obtain ${H_3}B{O_3}$ and the corresponding alcohol of the organic compound.
Thus, we can conclude that the answer of the question is (B).
Note:
Note that in hydroboration reaction, the hydrogen atom and hydroxyl group in the final product is syn to each other. Thus, this is syn-addition reaction. The product is the same as the anti-markovnikov addition product.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




