
How would the product of cis-stilbene look like after bromination?
Answer
559.5k+ views
Hint: The answer to this question lies in the fact of organic chemistry where halogenations substitutes the bromine atoms and forms the dibromo compound of cis-stilbene and the structural changes can be found by drawing the structures.
Complete step by step answer:
In the previous classes of organic chemistry, we have studied the basic named reactions and also several reactions like the addition reactions, substitution reactions, elimination reactions and many other.
- Let us see in detail about the bromination reaction and the final product obtained after the bromination.
Firstly let us see the structure of cis stilbene.
- Cis- stilbene is a diaryl ethane which consists of cis ethane bond that is substituted by a phenyl group on both the carbon atoms of double bond in the cis form.
- When this stilbene is subjected to bromination reaction, the nucleophile bromine attacks the double bond of alkene and forms the cyclic bromonium ion intermediate. The other bromine atoms approach from the opposite direction which then gives the racemic mixture of the products that is S,S and R,R configuration respectively.
This is as shown below,
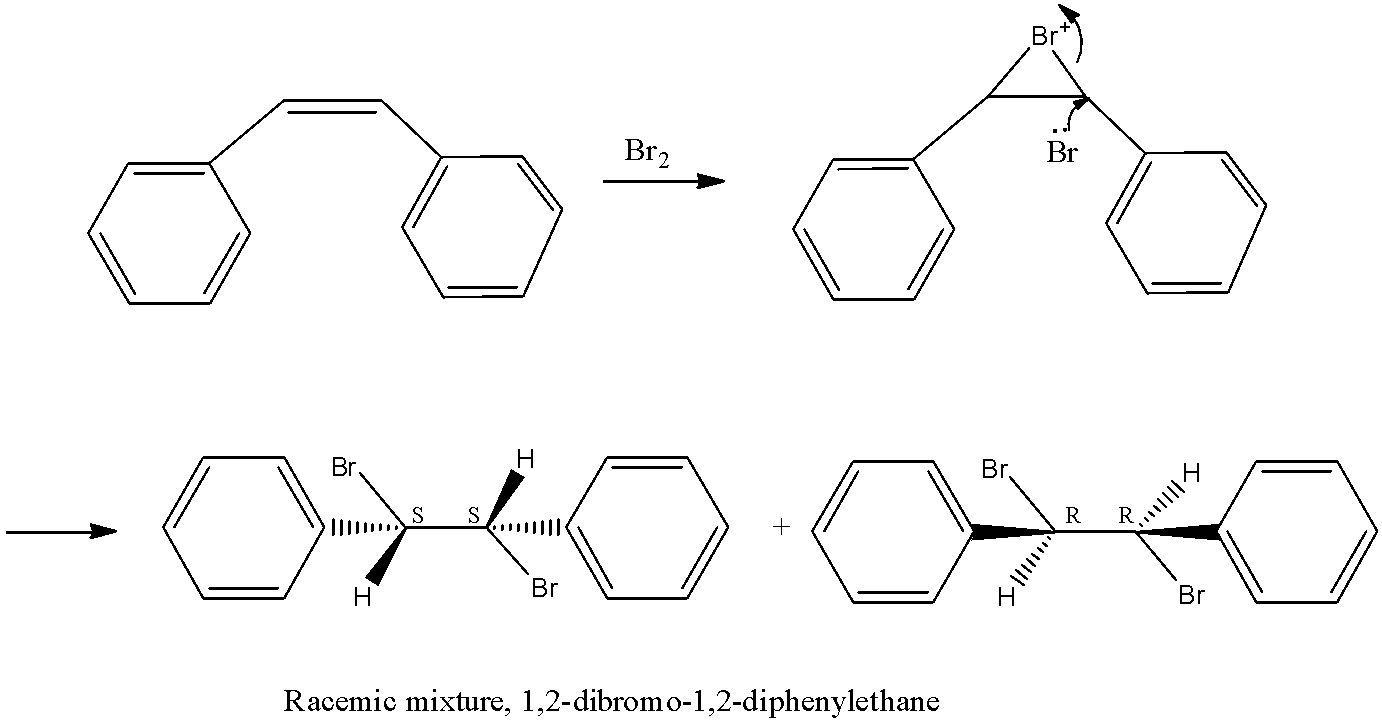
These products formed that is the racemic mixture formed by cis stilbene is confirmed by the structures drawn above and the picture would look like the structures shown above.
Note: Note that the properties of the cis and the trans stilbene varies and the bromination products for these two are different and it is not to be confused as cis stilbene gives the racemic mixture of the two enantiomers ad the trans stilbene yields the meso products.
Complete step by step answer:
In the previous classes of organic chemistry, we have studied the basic named reactions and also several reactions like the addition reactions, substitution reactions, elimination reactions and many other.
- Let us see in detail about the bromination reaction and the final product obtained after the bromination.
Firstly let us see the structure of cis stilbene.
- Cis- stilbene is a diaryl ethane which consists of cis ethane bond that is substituted by a phenyl group on both the carbon atoms of double bond in the cis form.
- When this stilbene is subjected to bromination reaction, the nucleophile bromine attacks the double bond of alkene and forms the cyclic bromonium ion intermediate. The other bromine atoms approach from the opposite direction which then gives the racemic mixture of the products that is S,S and R,R configuration respectively.
This is as shown below,

These products formed that is the racemic mixture formed by cis stilbene is confirmed by the structures drawn above and the picture would look like the structures shown above.
Note: Note that the properties of the cis and the trans stilbene varies and the bromination products for these two are different and it is not to be confused as cis stilbene gives the racemic mixture of the two enantiomers ad the trans stilbene yields the meso products.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




