
Product is:
MCPBA- Meta Chloroperbenzoic acid
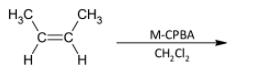
A)
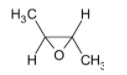
B)

C)
D)





Answer
548.1k+ views
Hint:
To answer this question, you must recall the reaction of alkenes with a peroxy acid. Meta- chloroperbenzoic acid is a peroxy carboxylic acid commonly referred to as mCPBA. It is a derivative of meta- chloro benzoic acid.
Complete step by step solution:
As the name suggests, mCPBA is a peroxy acid. A peroxy acid has an oxygen- oxygen bond. It resembles the structure of meta- chloro benzoic acid but has an extra oxygen atom. When reacted with an alkene, it forms epoxides. The reaction has a very key feature that the stereochemistry of the reactant is retained. This means, that if a cis alkene is reacted, a cis epoxide will be formed whereas, a trans alkene will result into the formation of a trans epoxide. The reaction is a stereo selective reaction.
In the question, the given alkene is a cis alkene with both the methyl groups on the same side. We know that the reaction is stereo selective and thus the epoxide formed will be of the same stereochemistry. The product thus formed will be
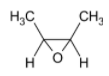
The correct option is B.
Note:
The reaction is known as Prilezhaev reaction. It proceeds through what is called as the butterfly mechanism. The reaction takes place through the formation of a transition state in which the plane of m-CPBA bisects the plane of the alkene with the oxygen- oxygen bond perpendicular to it. Due to this alignment, frontal orbital interactions occur. We can see this reaction as an electrophilic attack where the peroxy acid is an electrophile and the alkene is nucleophile.
To answer this question, you must recall the reaction of alkenes with a peroxy acid. Meta- chloroperbenzoic acid is a peroxy carboxylic acid commonly referred to as mCPBA. It is a derivative of meta- chloro benzoic acid.
Complete step by step solution:
As the name suggests, mCPBA is a peroxy acid. A peroxy acid has an oxygen- oxygen bond. It resembles the structure of meta- chloro benzoic acid but has an extra oxygen atom. When reacted with an alkene, it forms epoxides. The reaction has a very key feature that the stereochemistry of the reactant is retained. This means, that if a cis alkene is reacted, a cis epoxide will be formed whereas, a trans alkene will result into the formation of a trans epoxide. The reaction is a stereo selective reaction.
In the question, the given alkene is a cis alkene with both the methyl groups on the same side. We know that the reaction is stereo selective and thus the epoxide formed will be of the same stereochemistry. The product thus formed will be

The correct option is B.
Note:
The reaction is known as Prilezhaev reaction. It proceeds through what is called as the butterfly mechanism. The reaction takes place through the formation of a transition state in which the plane of m-CPBA bisects the plane of the alkene with the oxygen- oxygen bond perpendicular to it. Due to this alignment, frontal orbital interactions occur. We can see this reaction as an electrophilic attack where the peroxy acid is an electrophile and the alkene is nucleophile.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




