
What is the product formed when acetone heated in presence of Mg/Hg followed by hydrolysis?
A.
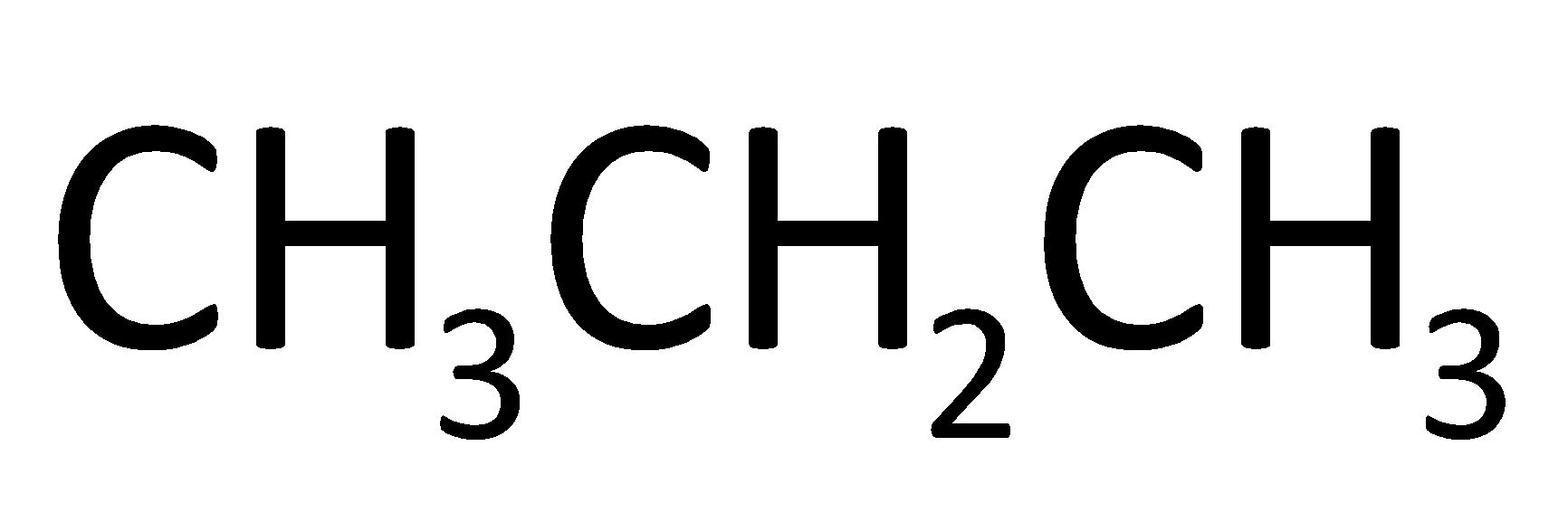
B.
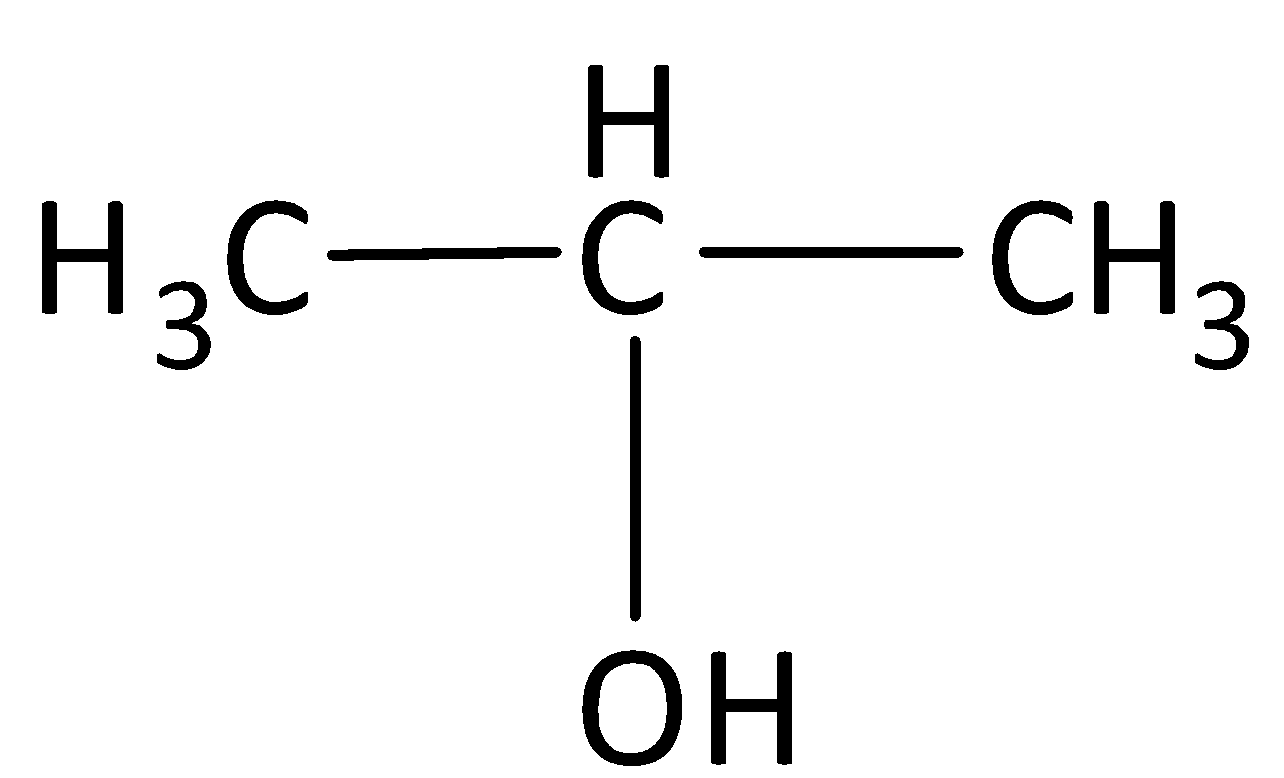
C.
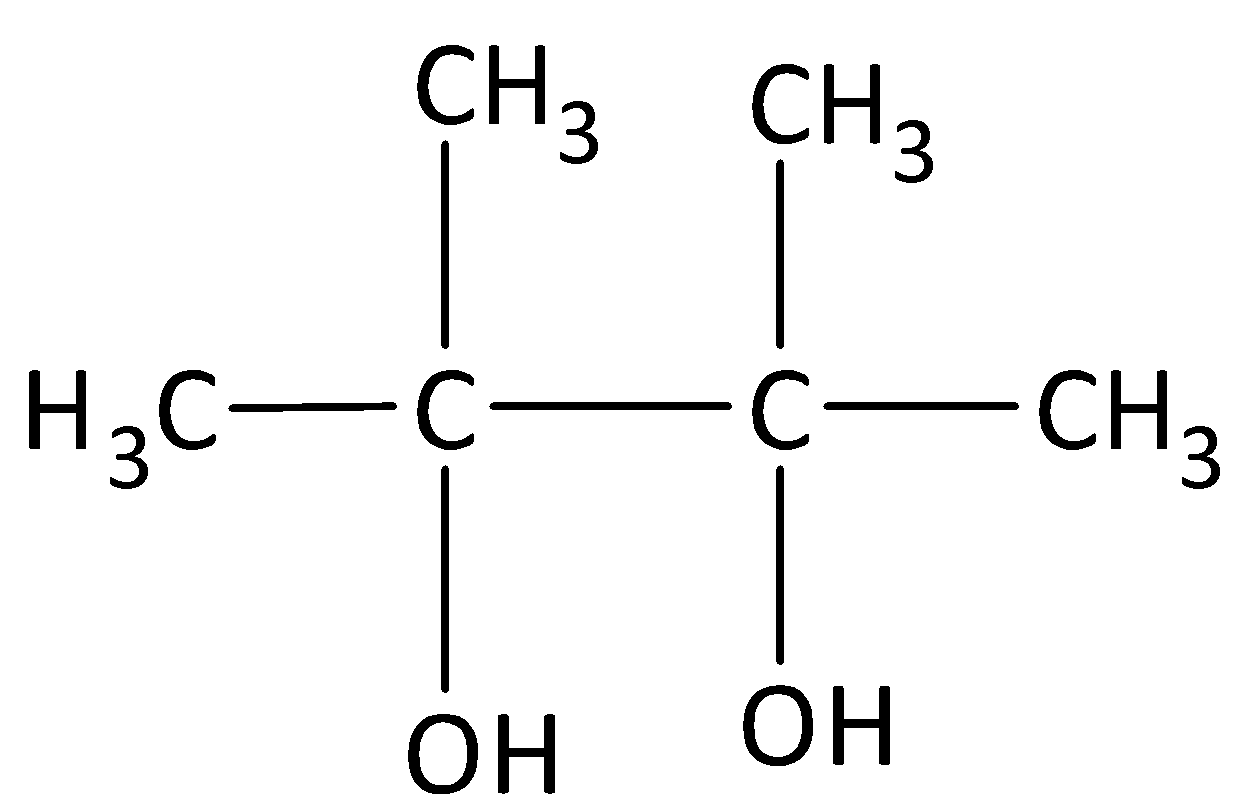
D.
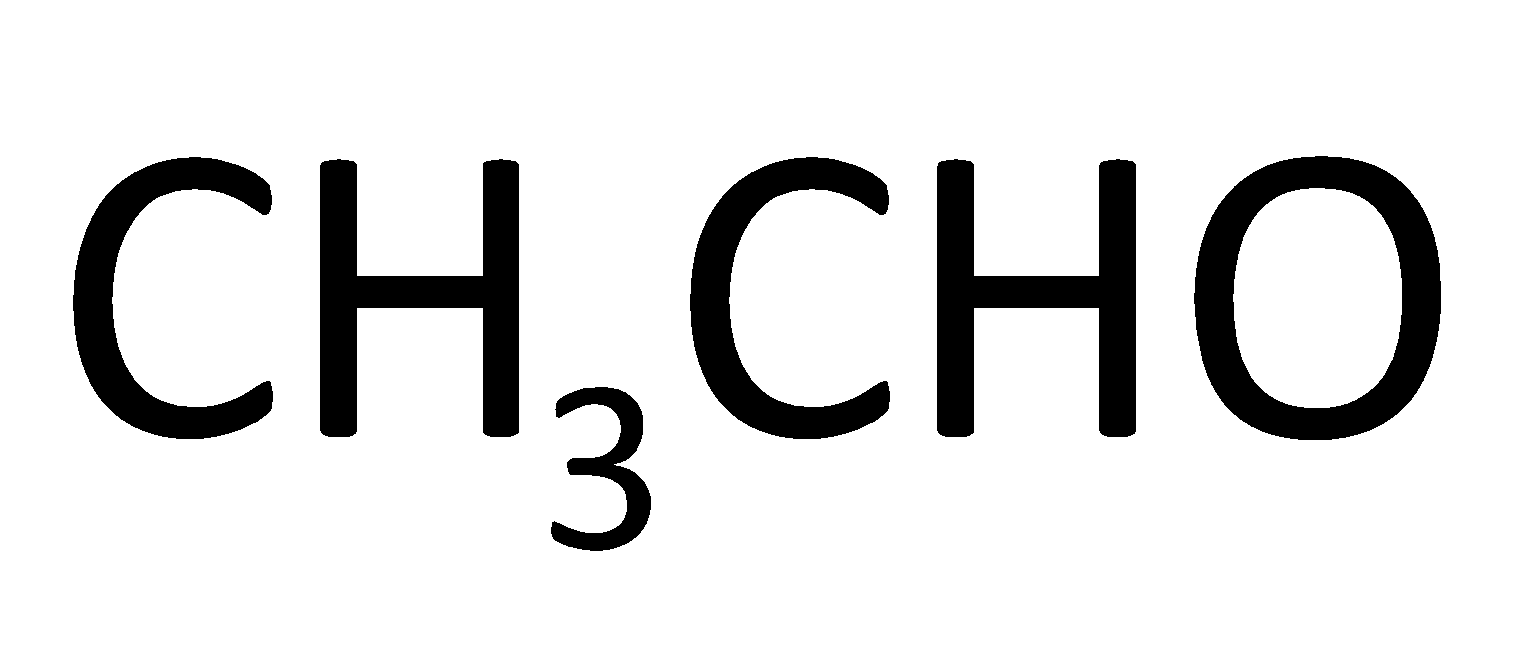
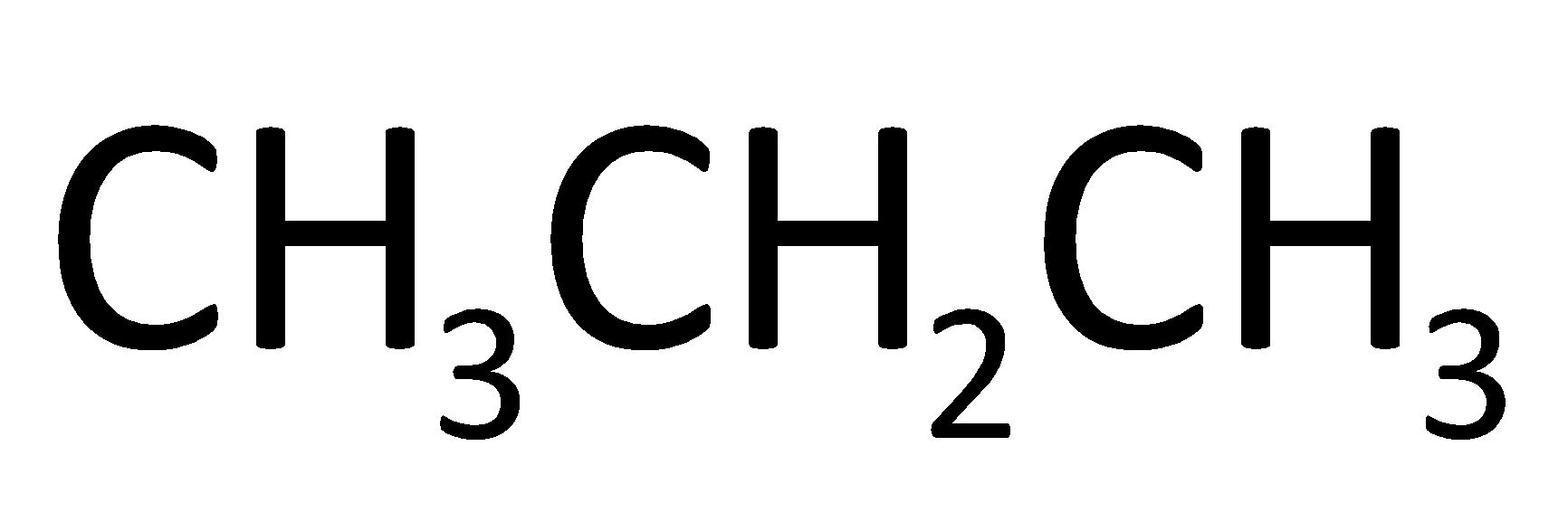


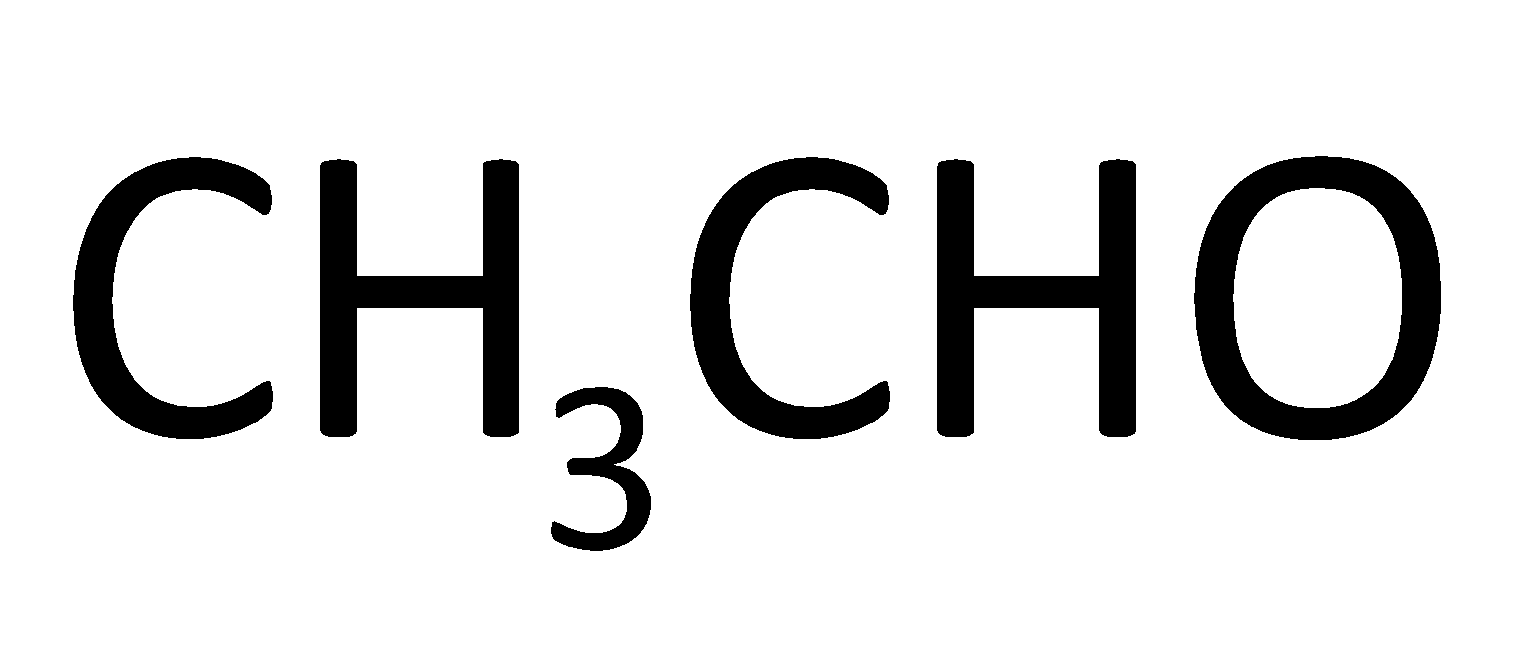
Answer
569.7k+ views
Hint: In pinacol coupling reaction, there is a formation of carbon-carbon between the carbonyl group of ketone or an aldehyde in the presence of electron donor such as Mg or Hg in a free radical process. The product of this reaction is vicinal diol.
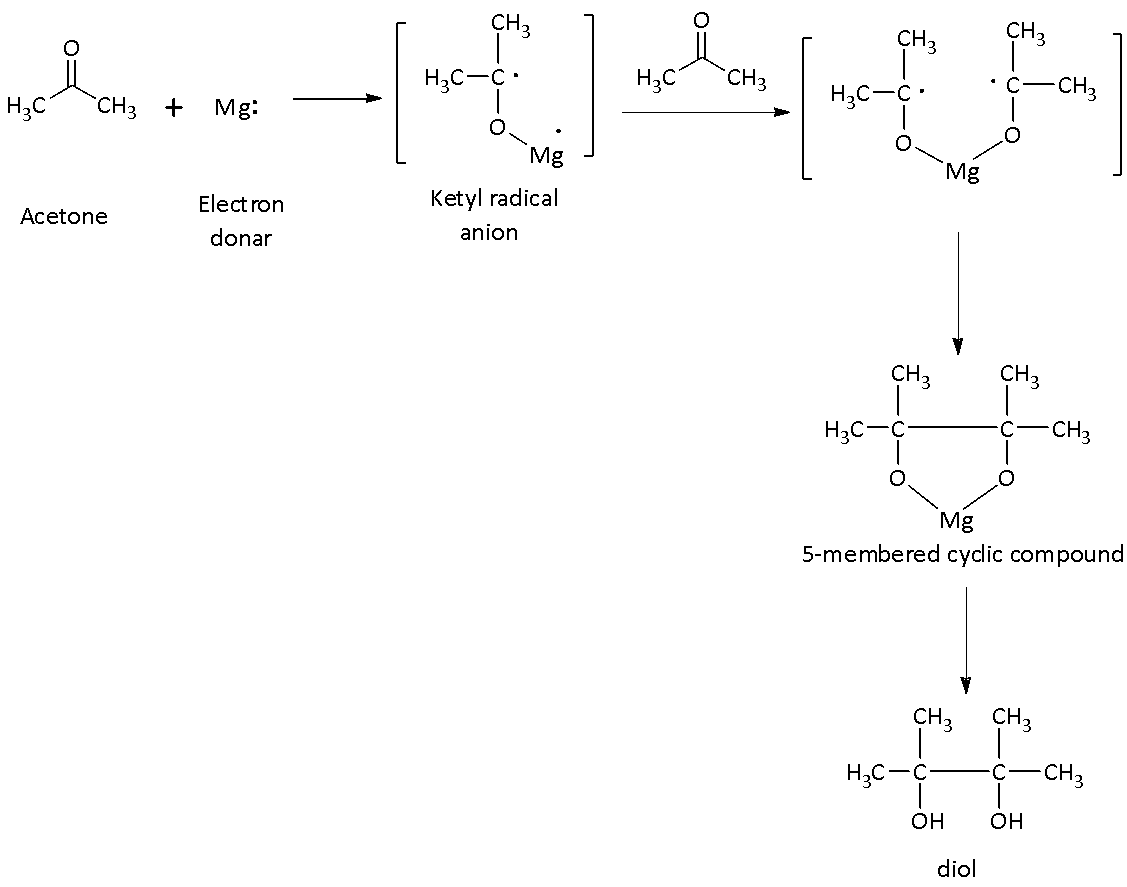
Complete step by step answer: When acetone is heated in the presence of Mg/Hg followed by hydrolysis reduces to gives pinacol which is named as \[{\rm{2,3 - dimethyl - 2,3 - diol}}\].
The mechanism of the formation of pinacol is as follows:
The first step is the one-electron reduction of the carbonyl group of the acetone by the reducing agent such as magnesium or mercury to a ketyl radical anion species.
In the next step, the ketyl radical anion, which is formed in the first step couples with another ketyl radical to form vicinal diol with deprotonated hydroxyl group.
In the third step, the addition of water (a proton donor) gives the diol.
So, the correct answer is “Option C”.
Note:
There is a formation of 5-membered cyclic compound with two oxygen atoms coordinated with the oxidized \[{\rm{M}}{{\rm{g}}^{{\rm{2 + }}}}\;{\rm{or}}\;{\rm{H}}{{\rm{g}}^{{\rm{2 + }}}}\] which broken up by adding water. McMurry reaction is a related reaction in which titanium(III) chloride or titanium(IV) chloride is used in place of Mg/Hg in conjunction with a reducing agent and gives metal-diol complex.
Complete step by step answer: When acetone is heated in the presence of Mg/Hg followed by hydrolysis reduces to gives pinacol which is named as \[{\rm{2,3 - dimethyl - 2,3 - diol}}\].
The mechanism of the formation of pinacol is as follows:
The first step is the one-electron reduction of the carbonyl group of the acetone by the reducing agent such as magnesium or mercury to a ketyl radical anion species.
In the next step, the ketyl radical anion, which is formed in the first step couples with another ketyl radical to form vicinal diol with deprotonated hydroxyl group.
In the third step, the addition of water (a proton donor) gives the diol.

So, the correct answer is “Option C”.
Note:
There is a formation of 5-membered cyclic compound with two oxygen atoms coordinated with the oxidized \[{\rm{M}}{{\rm{g}}^{{\rm{2 + }}}}\;{\rm{or}}\;{\rm{H}}{{\rm{g}}^{{\rm{2 + }}}}\] which broken up by adding water. McMurry reaction is a related reaction in which titanium(III) chloride or titanium(IV) chloride is used in place of Mg/Hg in conjunction with a reducing agent and gives metal-diol complex.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




