
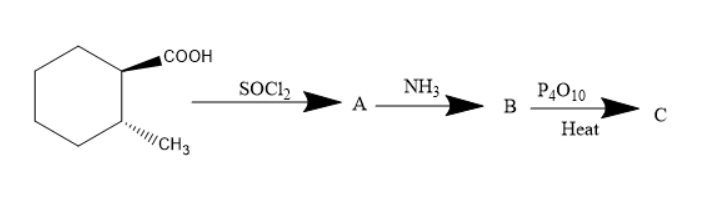
What is the product $C$ in the following reaction?
D) None of these




Answer
503.1k+ views
Hint: Carboxylic acids are acid functional groups and when treated with thionyl chloride forms acid chlorides. When acid chlorides treated with ammonia form amide compounds. When amide compounds treated with phosphorus oxides form, corresponding nitriles or cyanides are formed.
Complete answer:
Given compound is cyclohexyl 1-methyl 2-carboxylic acid. When this compound is treated with thionyl chloride the carboxylic acid group converts into an acyl group. The compound formed will be cyclohexyl 1-methyl 2-acetyl compound. When this compound is treated with ammonia the nucleophilic group in acetyl group i.e.., chlorine group will be replaced by amine group. Thus, the amide compound was formed.
When an amide compound is treated with phosphorus pentoxide the amide compound will be converted into cyanide compounds. Cyanide compounds are also known as nitriles.
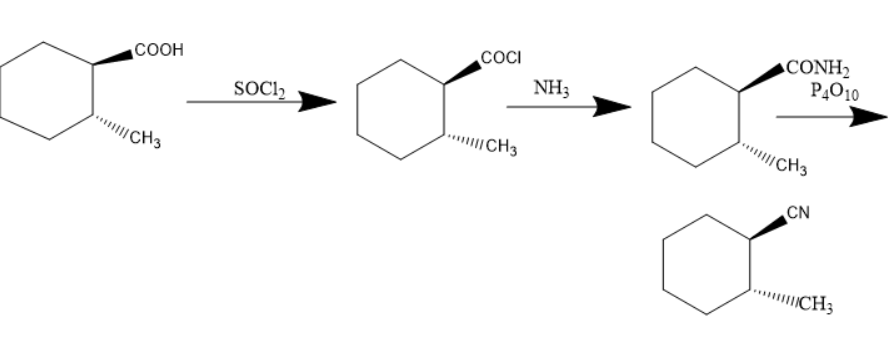
As the compound consists of wedged and dashed bonds, the product can be formed and will have dashed and wedged bonds.
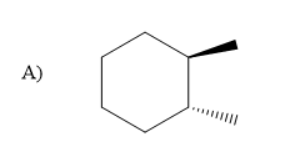
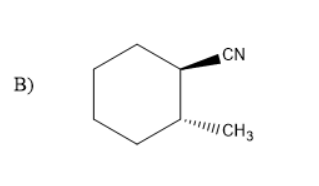
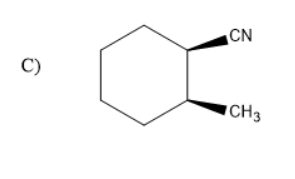
In the given compound there were two compounds with the substituents of cyanide and methyl on cyclohexane. But, in option C the two compounds were with wedged bonds. But, option B has methyl substituents and cyanide group on cyclohexane with one solid and wedged bond.
Note:
Thionyl chloride can be used as a chlorinating agent. Thus, chlorine was substituted in place of hydroxyl group in acids. The ammonia can be used as an amine donor. Phosphorous pentoxide can be used as a dehydrating agent and can be useful for the formation of nitriles or cyanides compounds.
Complete answer:
Given compound is cyclohexyl 1-methyl 2-carboxylic acid. When this compound is treated with thionyl chloride the carboxylic acid group converts into an acyl group. The compound formed will be cyclohexyl 1-methyl 2-acetyl compound. When this compound is treated with ammonia the nucleophilic group in acetyl group i.e.., chlorine group will be replaced by amine group. Thus, the amide compound was formed.
When an amide compound is treated with phosphorus pentoxide the amide compound will be converted into cyanide compounds. Cyanide compounds are also known as nitriles.

As the compound consists of wedged and dashed bonds, the product can be formed and will have dashed and wedged bonds.
In the given compound there were two compounds with the substituents of cyanide and methyl on cyclohexane. But, in option C the two compounds were with wedged bonds. But, option B has methyl substituents and cyanide group on cyclohexane with one solid and wedged bond.
Note:
Thionyl chloride can be used as a chlorinating agent. Thus, chlorine was substituted in place of hydroxyl group in acids. The ammonia can be used as an amine donor. Phosphorous pentoxide can be used as a dehydrating agent and can be useful for the formation of nitriles or cyanides compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




