
Product B is :
(a)-
(b)-
(c)-
(d)-
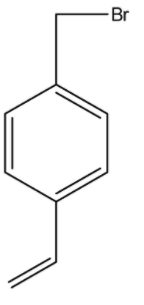





Answer
568.8k+ views
Hint: The reactant in the reaction is 4-Ethyl toluene or p-Ethyl toluene. NBS means the compound is N-Bromosuccinimide and 2 moles of NBS is used means two bromine atoms will be attached in the reactant compound. In the next step, the KCN is in which the cyanide group is the nucleophile and it will replace the bromine atom which is attached to the carbon atom having more number of hydrogen atoms.
Complete step by step answer:
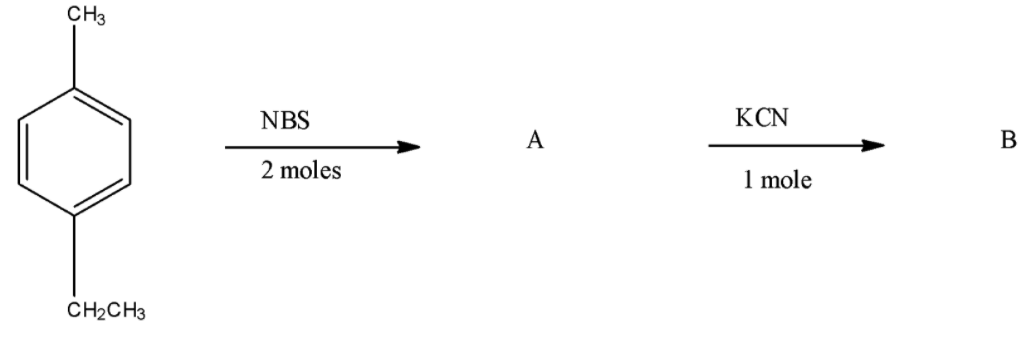
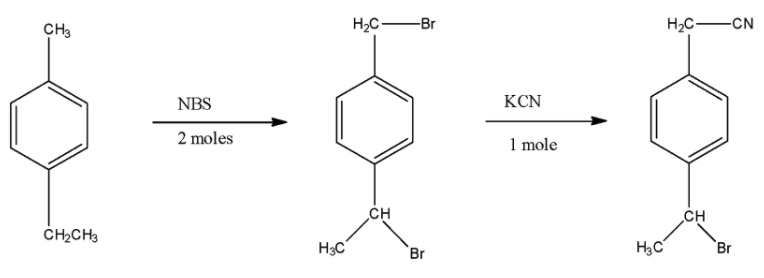
- The reactant molecule in the given reaction is 4-Ethyl toluene or p-Ethyl toluene means the ethyl group is present at the 4th position of the benzene ring and at the 1st position methyl group is present.
- Now, in the first reaction, the reactant molecule is treated with NBS, which means N-Bromosuccinimide, and this NBS is reacted for the addition of bromine atoms. As there are 2 moles used in this reaction, two bromine atoms will be substituted in the compound. One bromine atom will be attached to the carbon atom attached to the benzene ring at the 1st position and the second bromine will be attached to the carbon atom which is attached to the 4th atom of the benzene ring.
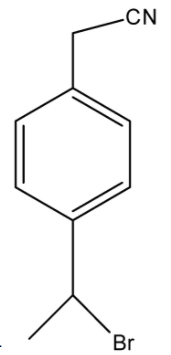
- Now, this compound is treated with KCN, in which the cyanide ($C{{N}^{-}}$) is a nucleophile that replaces the bromine atom which is attached to the carbon atom having a higher number of hydrogen atoms. The series of reaction is given below:
The correct answer is option “B” .
Note: The structure of N- Bromosuccinimide, is given below:
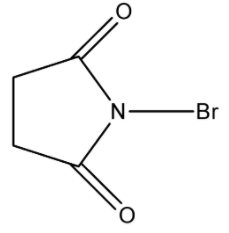
This reagent is used in many types of Electrophilic addition and substitution reaction as well as Nucleophilic addition and substitution reaction, and it is an excellent source of bromine atoms in the reaction.
Complete step by step answer:
- The reactant molecule in the given reaction is 4-Ethyl toluene or p-Ethyl toluene means the ethyl group is present at the 4th position of the benzene ring and at the 1st position methyl group is present.
- Now, in the first reaction, the reactant molecule is treated with NBS, which means N-Bromosuccinimide, and this NBS is reacted for the addition of bromine atoms. As there are 2 moles used in this reaction, two bromine atoms will be substituted in the compound. One bromine atom will be attached to the carbon atom attached to the benzene ring at the 1st position and the second bromine will be attached to the carbon atom which is attached to the 4th atom of the benzene ring.
- Now, this compound is treated with KCN, in which the cyanide ($C{{N}^{-}}$) is a nucleophile that replaces the bromine atom which is attached to the carbon atom having a higher number of hydrogen atoms. The series of reaction is given below:

The correct answer is option “B” .
Note: The structure of N- Bromosuccinimide, is given below:

This reagent is used in many types of Electrophilic addition and substitution reaction as well as Nucleophilic addition and substitution reaction, and it is an excellent source of bromine atoms in the reaction.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




