
What will produce nitrobenzene on reaction with $Sn/HCl$ ?
Answer
507.3k+ views
Hint: We have to know that, consider a self-assertive fragrant nitro compound and attempt to compose the response instrument with $Sn/HCl$ . Tin responds with $HCl$ and framed tin (IV) chloride. Henceforth, we can say that ${H^ + }$ particles are discharges, which respond with the nitro utilitarian gathering of the sweet-smelling compound.
Complete answer:
We have to see that nitration of fragrant mixtures is utilized to add nitrogen particles to the aromatic compound as nitro $ - N{O_2}$ bunch. The nitrating combination comprises hot concentrated nitric corrosive additionally called hot seething nitric corrosive furthermore, concentrated sulphuric corrosive.
Sulphuric corrosive is a more grounded corrosive than nitric corrosive. Sulphuric corrosive protonated nitrogen present in the nitric corrosive. This prompts the arrangement of nitroso particles $NO_2^ + $ .
Nitroso particles are then added to the benzene ring through electrophilic replacement response. This is the manner by which fragrant nitro compounds are shaped.
We will presently consider a sweet-smelling nitro compound and compose its response with $Sn/HCl$ .
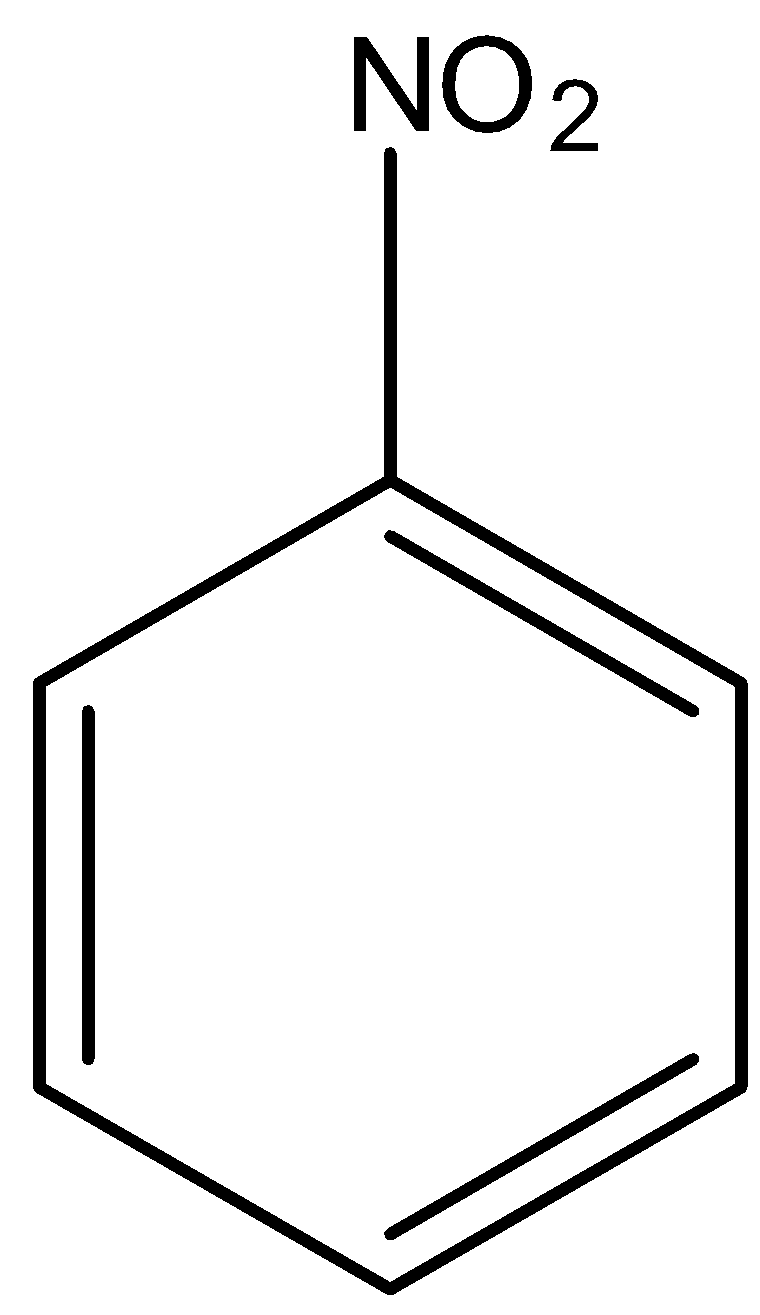
Tin responds with hydrochloric corrosive to shape Tin (IV) chloride. The ${H^ + }$ particles present respond with the nitro gathering of the fragrant compound. The oxygen from the nitro bunch is delivered as water particles. Instead of oxygen molecules, hydrogen gets subbed prompting the development of aromatic amine.
The balanced chemical equation is given below,
$2{C_6}{H_5}N{O_2} + 3Sn + 12HCl \to 2{C_6}{H_5}N{H_2} + 3SnC{l_4} + 4{H_2}O$
The item framed is aniline, which is an aromatic essential amine.
Note:
We have to know that electrophilic aromatic-replacement is a natural response where the hydrogen that is joined to a natural framework is supplanted by approaching electrophile. The portion of the significant electrophilic aromatic-replacement, incorporates fragrant nitration.
Complete answer:
We have to see that nitration of fragrant mixtures is utilized to add nitrogen particles to the aromatic compound as nitro $ - N{O_2}$ bunch. The nitrating combination comprises hot concentrated nitric corrosive additionally called hot seething nitric corrosive furthermore, concentrated sulphuric corrosive.
Sulphuric corrosive is a more grounded corrosive than nitric corrosive. Sulphuric corrosive protonated nitrogen present in the nitric corrosive. This prompts the arrangement of nitroso particles $NO_2^ + $ .
Nitroso particles are then added to the benzene ring through electrophilic replacement response. This is the manner by which fragrant nitro compounds are shaped.
We will presently consider a sweet-smelling nitro compound and compose its response with $Sn/HCl$ .

Tin responds with hydrochloric corrosive to shape Tin (IV) chloride. The ${H^ + }$ particles present respond with the nitro gathering of the fragrant compound. The oxygen from the nitro bunch is delivered as water particles. Instead of oxygen molecules, hydrogen gets subbed prompting the development of aromatic amine.
The balanced chemical equation is given below,
$2{C_6}{H_5}N{O_2} + 3Sn + 12HCl \to 2{C_6}{H_5}N{H_2} + 3SnC{l_4} + 4{H_2}O$
The item framed is aniline, which is an aromatic essential amine.
Note:
We have to know that electrophilic aromatic-replacement is a natural response where the hydrogen that is joined to a natural framework is supplanted by approaching electrophile. The portion of the significant electrophilic aromatic-replacement, incorporates fragrant nitration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




