
How many primary amines are possible for the formula ${{\rm{C}}_{\rm{4}}}{{\rm{H}}_{{\rm{11}}}}{\rm{N}}$?
(a) 4
(b) 2
(c) 3
(d )5
Answer
573.6k+ views
Hint: We know that amine is an important class of organic compounds which is derived by replacement of one or more atoms of hydrogen of ammonia by alkyl or aryl groups. Naturally amines are present in vitamins, proteins and hormones. Also in some synthetic drugs and polymers amine is present. The structure of amine is ${\rm{R}} - {\rm{N}}{{\rm{H}}_{\rm{2}}}$ if one hydrogen atom is replaced.
Complete step by step solution:
Let’s understand the types of amine in detail. Amines are of three types, primary, secondary or tertiary.
Primary amine is the amine that is formed by replacing one hydrogen atom of ammonia by an alkyl or aryl group.
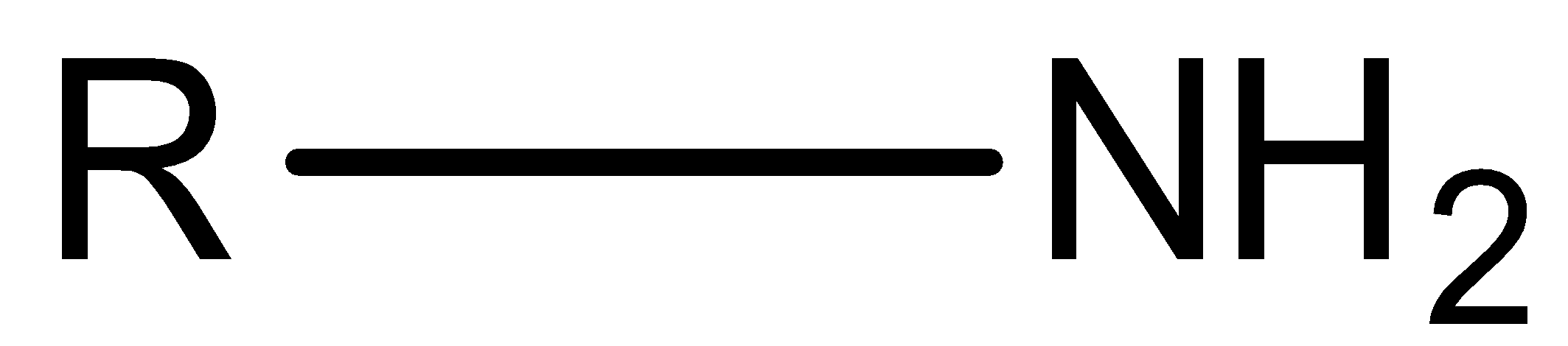
Secondary amine is the amine in which two hydrogen atoms of ammonia are replaced by two alkyl or aryl groups.

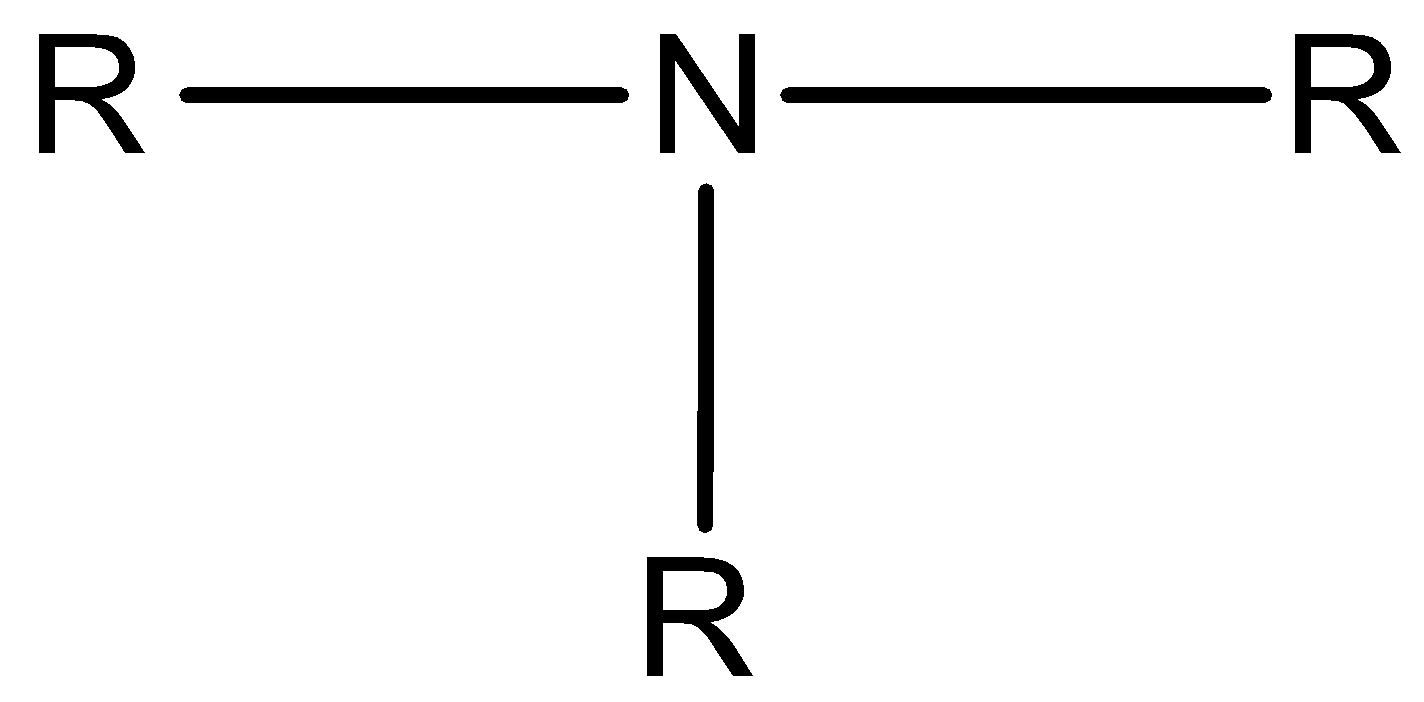
Tertiary amine is the amine in which all three hydrogen atoms of ammonia are replaced by three alkyl or aryl groups.
Now, come to the question. Here, we have to check the primary amines possible for ${{\rm{C}}_{\rm{4}}}{{\rm{H}}_{{\rm{11}}}}{\rm{N}}$.
There are four carbon atoms in the molecular formula. So, first primary amine of ${{\rm{C}}_{\rm{4}}}{{\rm{H}}_{{\rm{11}}}}{\rm{N}}$ is,
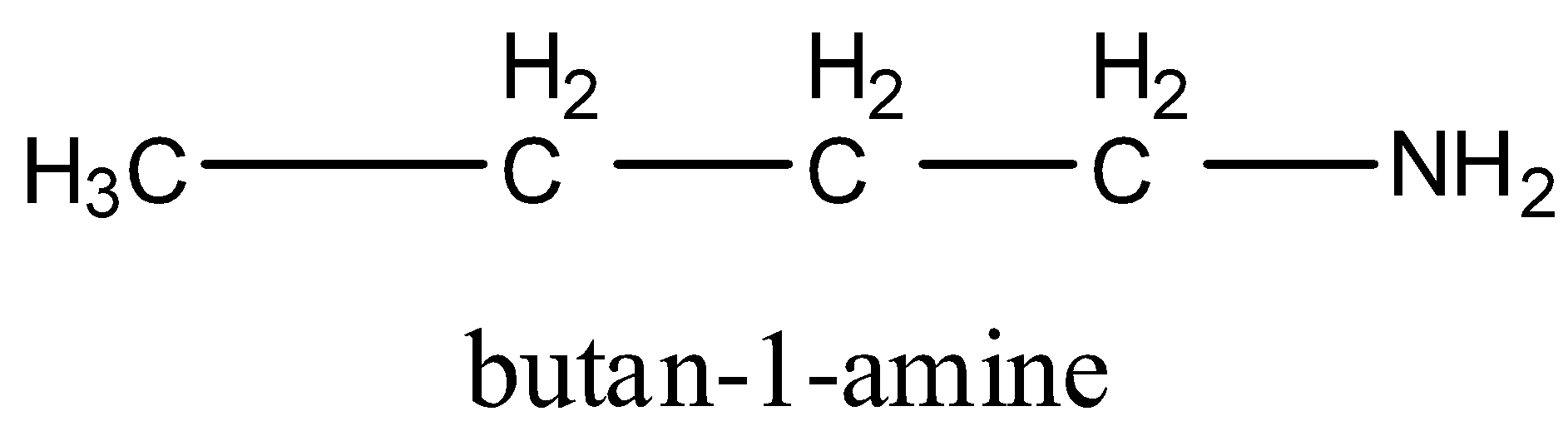
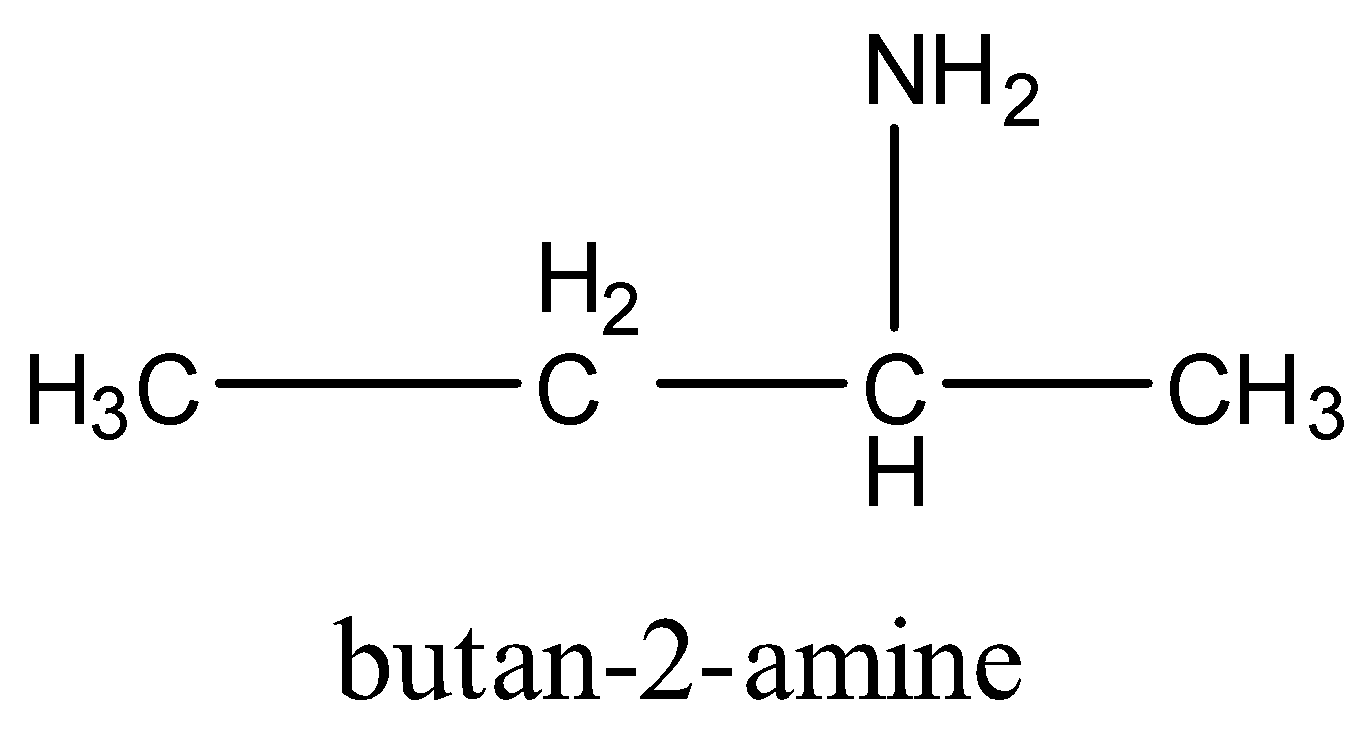
2nd primary amine is,
3rd primary amine is,
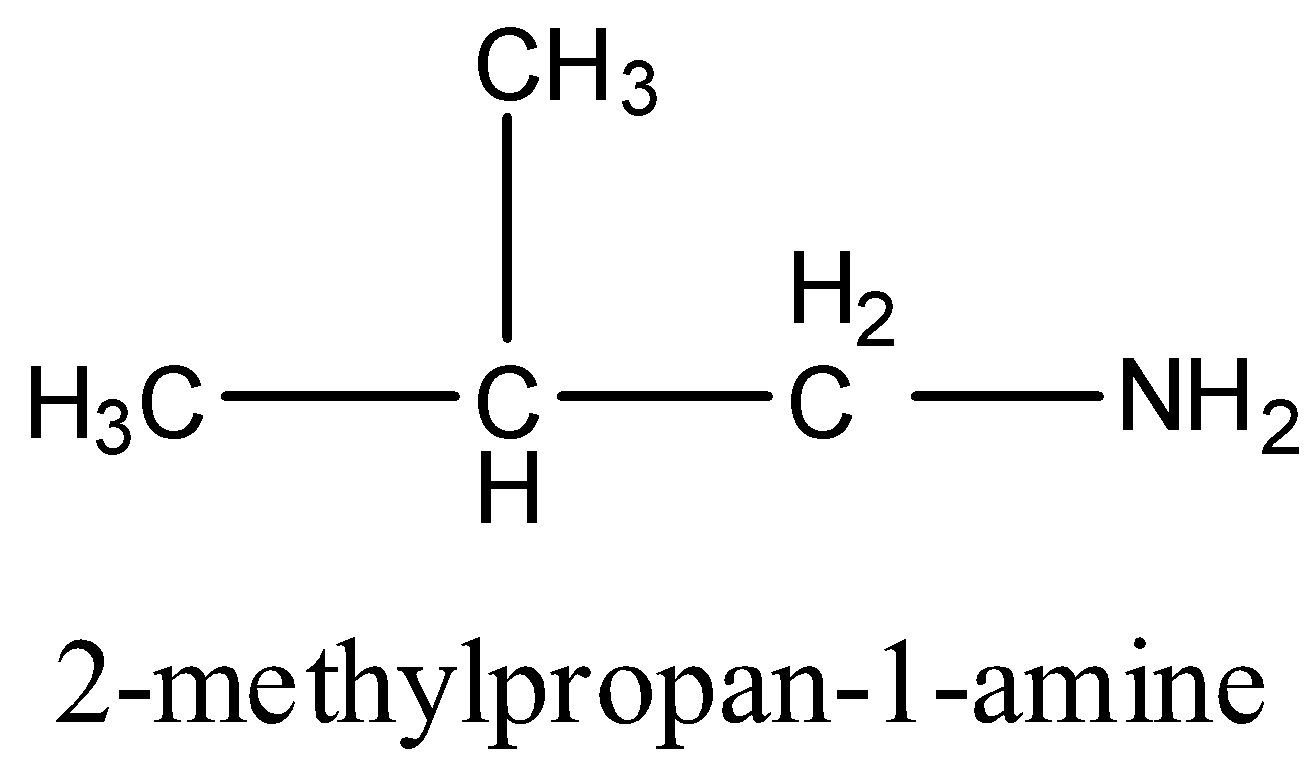
4th primary amine is,
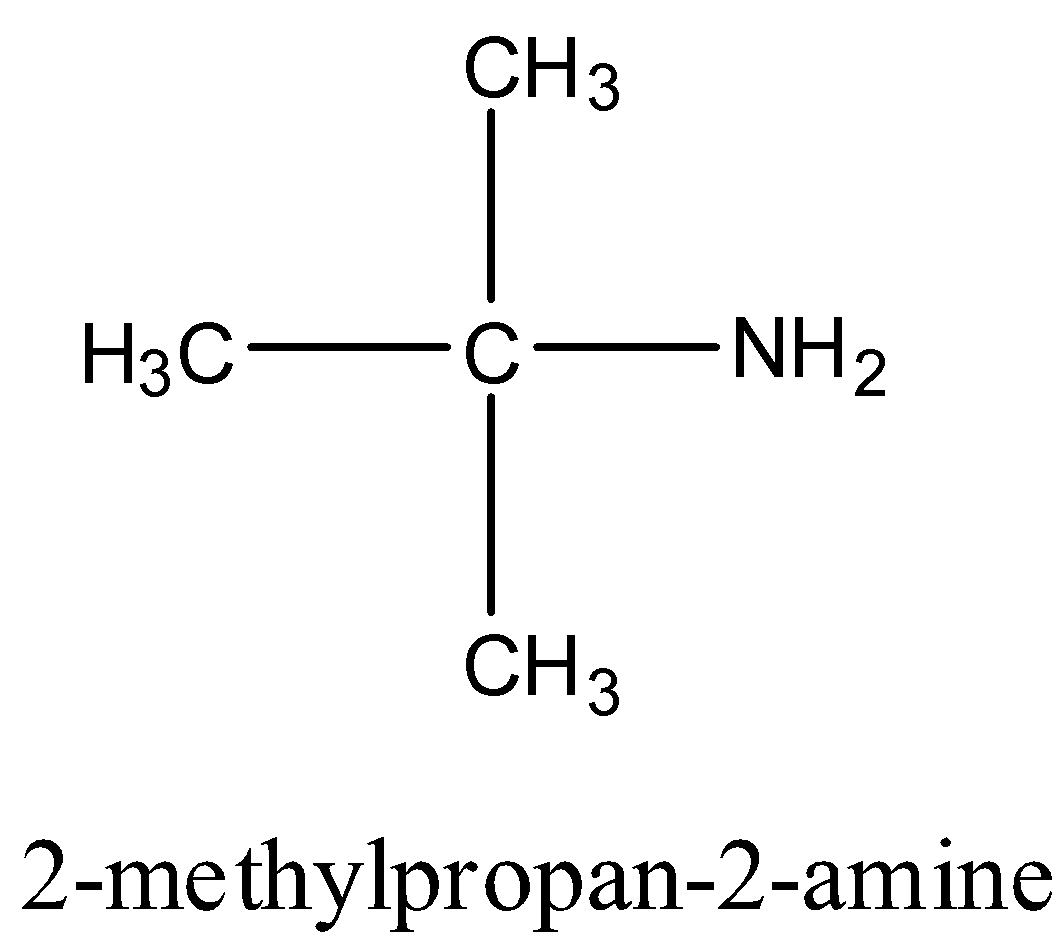
Therefore, four primary amines are possible for the molecular formula ${{\rm{C}}_{\rm{4}}}{{\rm{H}}_{{\rm{11}}}}{\rm{N}}$.
So, the correct answer is Option A.
Note: When aromatic or aliphatic primary amine is heated with ethanolic potassium hydroxide and chloroform, carbylamines or isocyanides form. This reaction is not shown by secondary and tertiary amines. So, this reaction is used as a test to identify primary amines.
$R-NH_{2}+CHCl_{3}+3KOH\overset{Heat}{\rightarrow}R-NC+3KCl+3H_{2}O$
Complete step by step solution:
Let’s understand the types of amine in detail. Amines are of three types, primary, secondary or tertiary.
Primary amine is the amine that is formed by replacing one hydrogen atom of ammonia by an alkyl or aryl group.
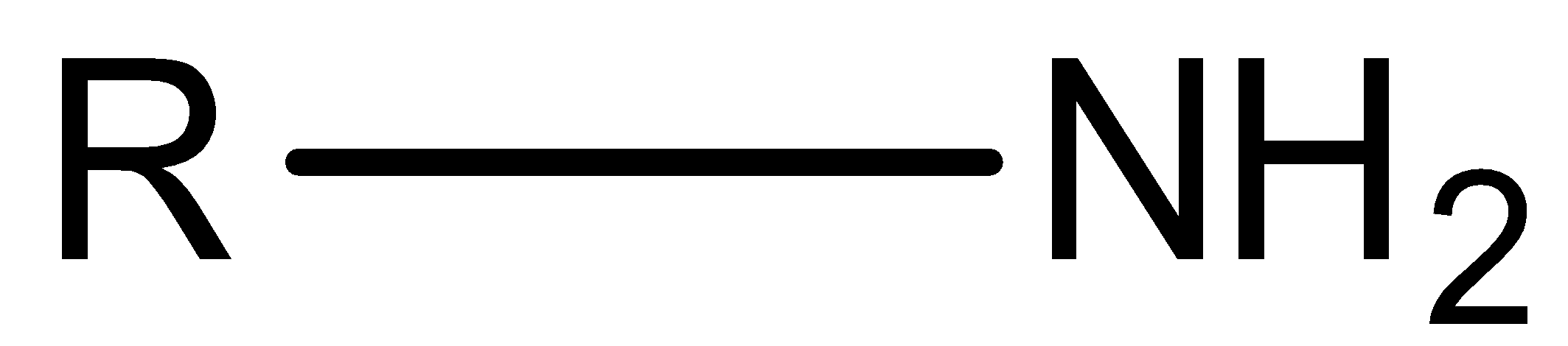
Secondary amine is the amine in which two hydrogen atoms of ammonia are replaced by two alkyl or aryl groups.

Tertiary amine is the amine in which all three hydrogen atoms of ammonia are replaced by three alkyl or aryl groups.

Now, come to the question. Here, we have to check the primary amines possible for ${{\rm{C}}_{\rm{4}}}{{\rm{H}}_{{\rm{11}}}}{\rm{N}}$.
There are four carbon atoms in the molecular formula. So, first primary amine of ${{\rm{C}}_{\rm{4}}}{{\rm{H}}_{{\rm{11}}}}{\rm{N}}$ is,
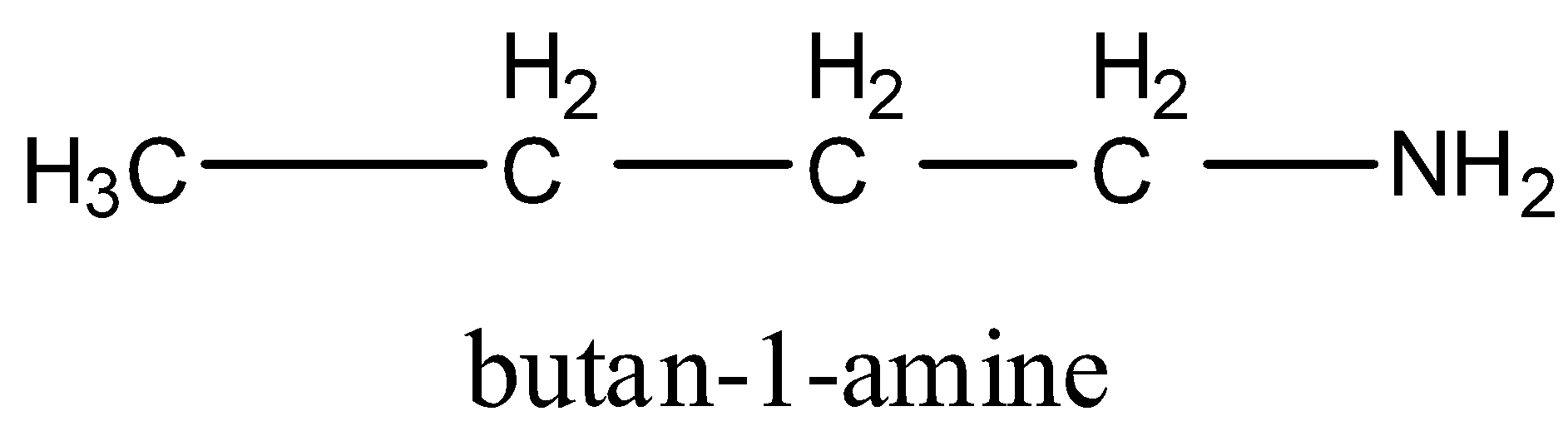
2nd primary amine is,

3rd primary amine is,

4th primary amine is,

Therefore, four primary amines are possible for the molecular formula ${{\rm{C}}_{\rm{4}}}{{\rm{H}}_{{\rm{11}}}}{\rm{N}}$.
So, the correct answer is Option A.
Note: When aromatic or aliphatic primary amine is heated with ethanolic potassium hydroxide and chloroform, carbylamines or isocyanides form. This reaction is not shown by secondary and tertiary amines. So, this reaction is used as a test to identify primary amines.
$R-NH_{2}+CHCl_{3}+3KOH\overset{Heat}{\rightarrow}R-NC+3KCl+3H_{2}O$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




