
Predict the product formed in the following reaction.
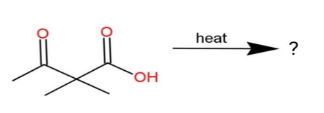
A. 3-methyl-2-butanone
B. 3, 3-dimethylmalonic acid
C. propane
D. 3, 3-dimethyl-2, 4-pentanedione

Answer
533.1k+ views
Hint: When an organic compound contains a ketonic group along with a carboxylic group, then it is called as a keto acid. The naming of keto acids can also be done as oxo alkanoic acids.
Complete step by step answer: We have been given a compound which is subjected to heat, and we have to find the product obtained. The compound contains a keto acid, with a 4 carbon chain along with 2 methyl branches. So, the name of this compound is 2,2-dimethyl-3-oxobutanoic acid. This keto acid when subjected to heat converts into di carboxylic acid, which is 3, 3-dimethylmalonic acid . The reaction is as,
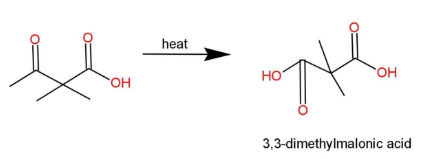
The reaction forms di carboxylic acid, as by heating of this keto acid, catalytic oxidation occurs, which creates an intermediate of carbanion, that has a hydroxyl group attached to form a stable 3, 3-dimethylmalonic acid.
Additional information: Keto acids are of various types, like alpha keto acids, that contain a keto group adjacent to the carboxyl group. Beta-keto acids that have a ketone group placed at the second carbon from the carboxyl group.
Hence, the reaction gives 3, 3-dimethylmalonic acid, so, option B is correct.
Note: Keto acids on heating have a removal of carboxyl ions. But here it is converted to 3, 3-dimethylmalonic acid, due to the compound being beta- keto acid heated at high temperature range produces an enol intermediate, hence the product here.
Complete step by step answer: We have been given a compound which is subjected to heat, and we have to find the product obtained. The compound contains a keto acid, with a 4 carbon chain along with 2 methyl branches. So, the name of this compound is 2,2-dimethyl-3-oxobutanoic acid. This keto acid when subjected to heat converts into di carboxylic acid, which is 3, 3-dimethylmalonic acid . The reaction is as,

The reaction forms di carboxylic acid, as by heating of this keto acid, catalytic oxidation occurs, which creates an intermediate of carbanion, that has a hydroxyl group attached to form a stable 3, 3-dimethylmalonic acid.
Additional information: Keto acids are of various types, like alpha keto acids, that contain a keto group adjacent to the carboxyl group. Beta-keto acids that have a ketone group placed at the second carbon from the carboxyl group.
Hence, the reaction gives 3, 3-dimethylmalonic acid, so, option B is correct.
Note: Keto acids on heating have a removal of carboxyl ions. But here it is converted to 3, 3-dimethylmalonic acid, due to the compound being beta- keto acid heated at high temperature range produces an enol intermediate, hence the product here.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




