
Predict the correct intermediate and the product in the following reaction:
${\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}} \equiv {\text{CH}}\xrightarrow[{{\text{HgS}}{{\text{O}}_{\text{4}}}}]{{{{\text{H}}_{\text{2}}}{\text{O,}}{{\text{H}}_2}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{Intermediate (A)}} \to {\text{Product}}\,{\text{(B)}}$
A.
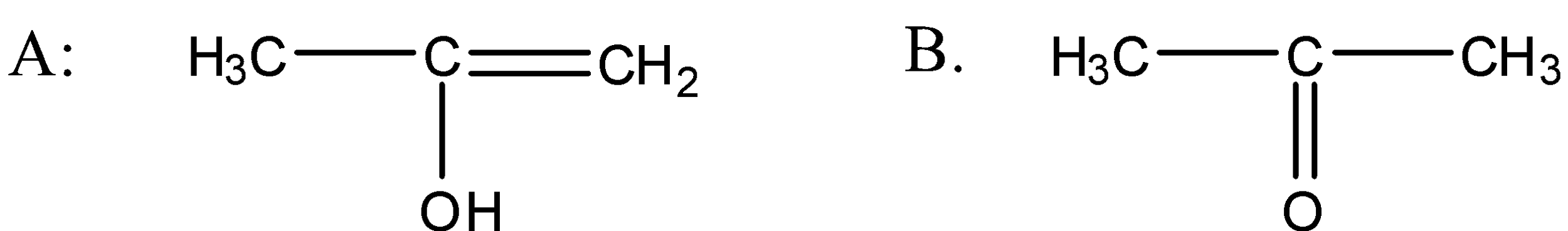
B.
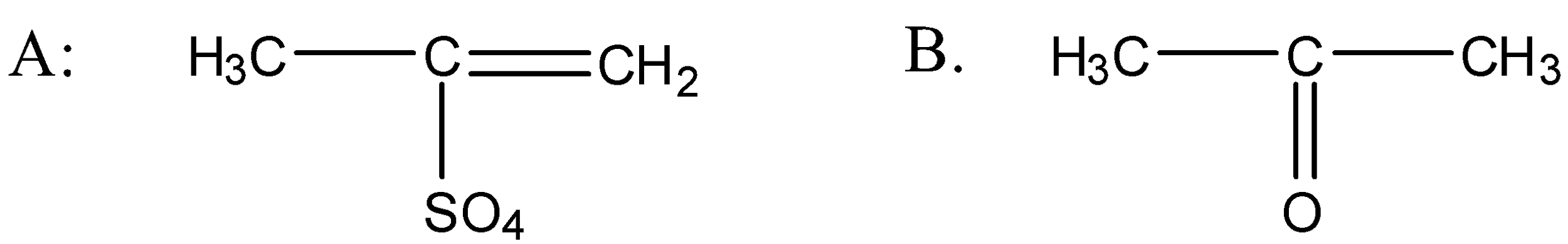
C.
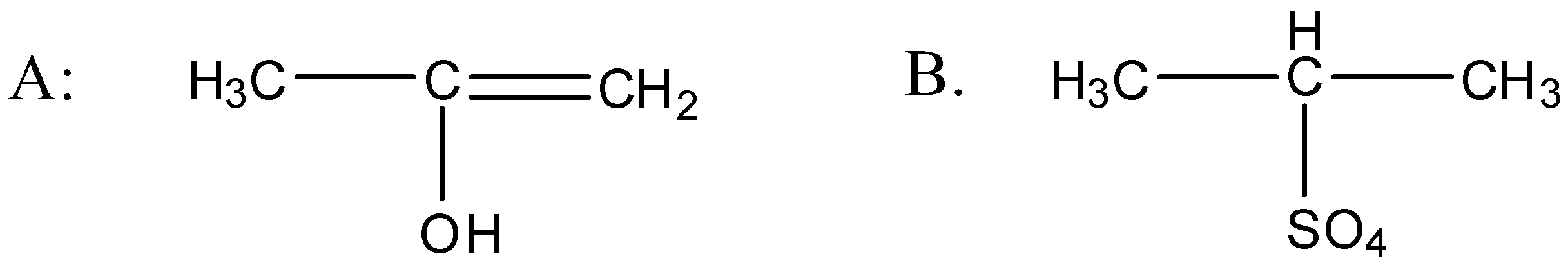
D.
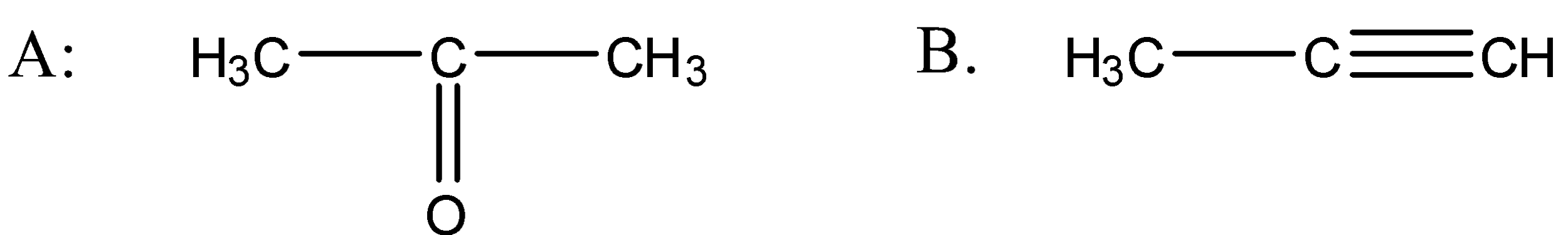
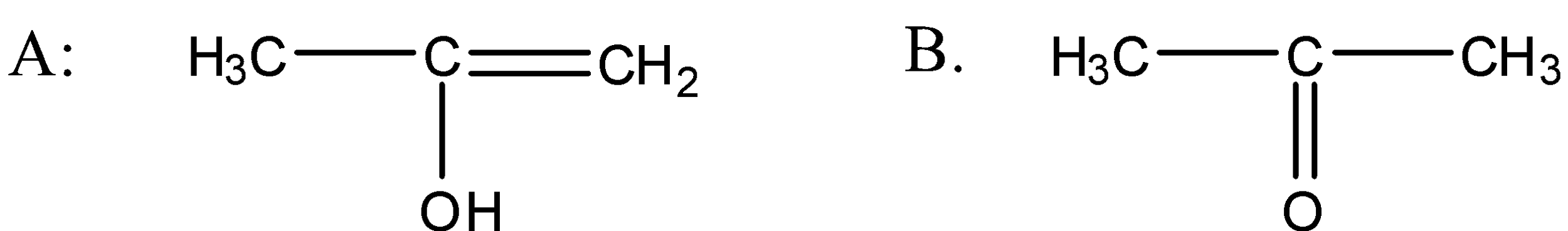

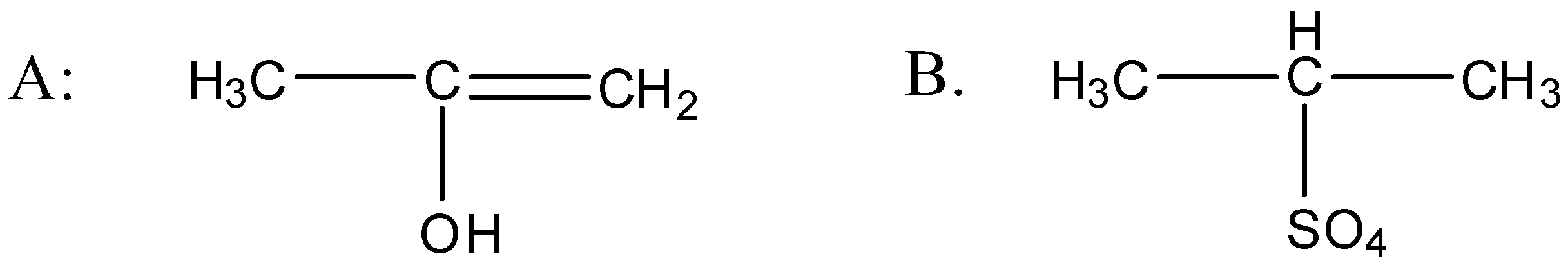
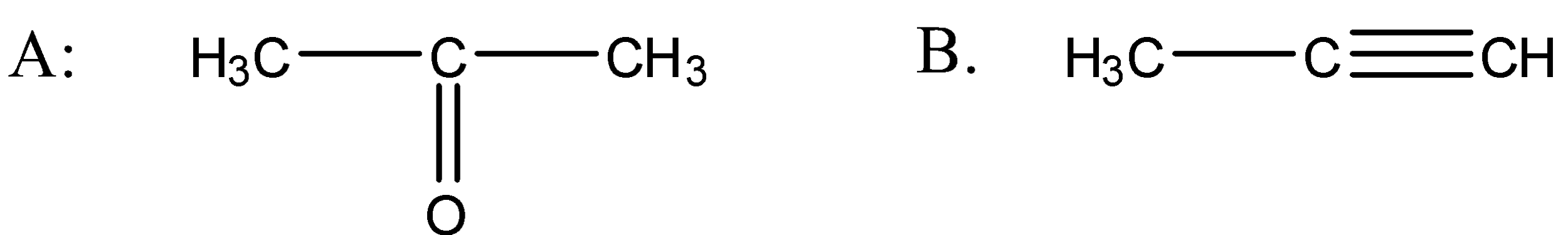
Answer
548.4k+ views
Hint: Ethyne is also known as acetylene which is an organic compound. It consists of two carbons that are bonded by triple bonds. The formation of ethyne takes place by partial combustion of methane. The other method for preparation of ethyne is by hydrolysing the compound called calcium carbide.
Complete step by step answer:
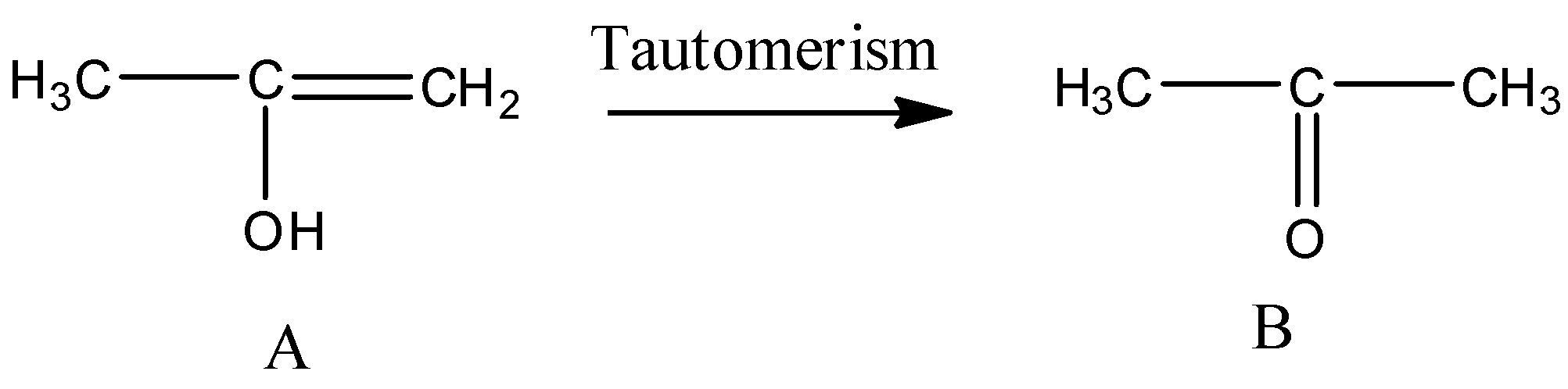
So when the propyne is treated with water, sulphuric acid and mercury sulphate the formation of an alcohol takes place and that alcohol has the following structure:
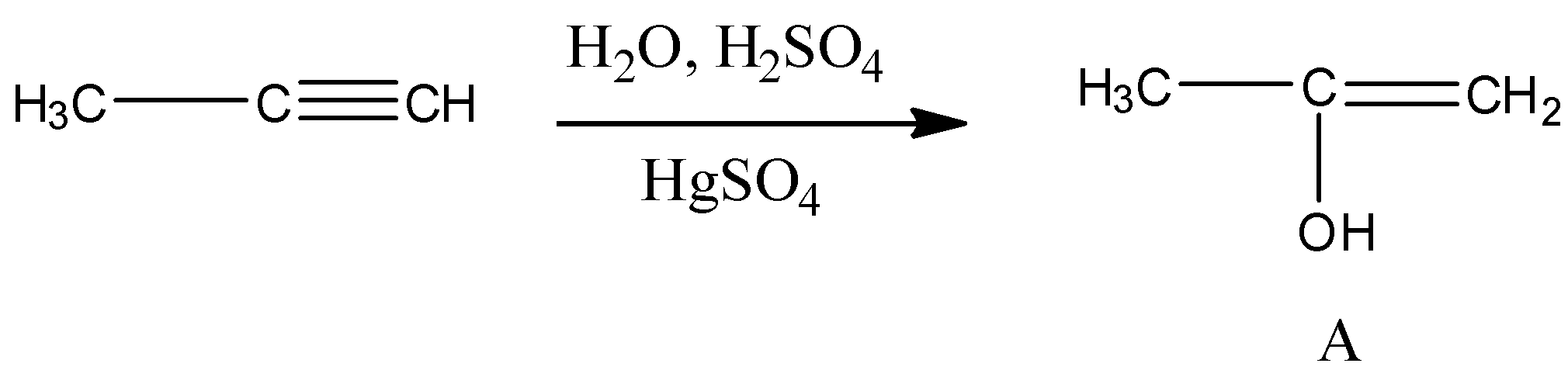
So the above alcohol is the intermediate. When this intermediate undergoes tautomerism then forms the ketone which is the final product. The product structure of ketone is the following:
So the correct answer for this question is option a.
The molar mass of ethyne is considered as 26.038 grams per mole. Under the standard conditions ethyne is a gas which is colourless and does not have any distinct odour. It tends to be slightly soluble in water. it has a shape of linear.
Hence, option A is the correct answer.
Note: Ethyne has the tendency to be burned with a hot flame so it is used as an oxyacetylene gas welding. It helps in the production of portable lightning too. It helps in the manufacturing of polyethene plastics. It tends to be converted into ethene under the process of semi hydrogenation.
Complete step by step answer:
So when the propyne is treated with water, sulphuric acid and mercury sulphate the formation of an alcohol takes place and that alcohol has the following structure:

So the above alcohol is the intermediate. When this intermediate undergoes tautomerism then forms the ketone which is the final product. The product structure of ketone is the following:

So the correct answer for this question is option a.
The molar mass of ethyne is considered as 26.038 grams per mole. Under the standard conditions ethyne is a gas which is colourless and does not have any distinct odour. It tends to be slightly soluble in water. it has a shape of linear.
Hence, option A is the correct answer.
Note: Ethyne has the tendency to be burned with a hot flame so it is used as an oxyacetylene gas welding. It helps in the production of portable lightning too. It helps in the manufacturing of polyethene plastics. It tends to be converted into ethene under the process of semi hydrogenation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




