
How can I predict the bond angles for $IF_{4}^{-}$ ?
Answer
564.6k+ views
Hint: In $IF_{4}^{-}$, there is one negative charge which means that there is an addition of one electron to the total valence electron in the molecule. There are four fluorine atoms attached to the iodine so there are four covalent bonds.
Complete answer:
For calculating the bond angle of the molecule then we have to predict the structure of the molecule. First, we have to find the number of valence electrons in the molecule. In $IF_{4}^{-}$, there are two atoms i.e., iodine and fluorine, and both of them are the elements belongs to group 17 so, they both have 7 electrons in their valence shell and there is one negative charge which means that there is the addition of one electron to total valence electron in the molecule. Therefore, the total number of valence electrons are:
$7+7+7+7+7+1=36$
There are four fluorine atoms attached to the iodine so, there are four covalent bonds and all the fluorine atoms will have three lone pairs. This constitutes 28 electrons and rest 4 electrons constitute the two lone pairs around the iodine atom. So, the coordination number of iodine will be 6 in which 4 are covalent bonds and 2 lone pairs. The shape will be a square planar in which all the corners are occupied by the fluorine atoms, one lone pair is above the plane, and one lone pair is below the plane. The structure is given below:
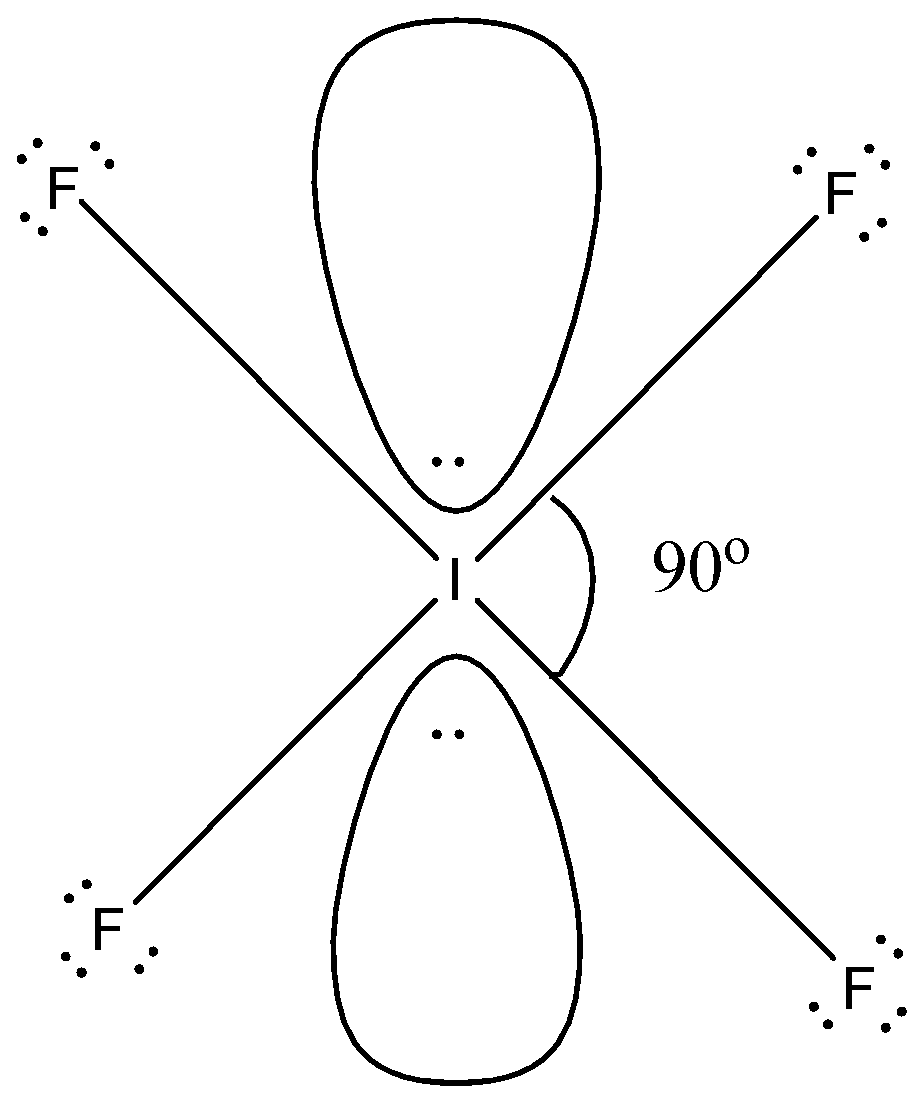
So, in square planar structure, the bond angle is ${{90}^{\circ }}$ therefore, bond angle in $IF_{4}^{-}$ will be ${{90}^{\circ }}$.
Note:
There is no repulsion between the lone pair and bond pair in the $IF_{4}^{-}$ because all the bond in $IF_{4}^{-}$ are in one plane but the lone pairs are above and below the planes so, there is no repulsion.
Complete answer:
For calculating the bond angle of the molecule then we have to predict the structure of the molecule. First, we have to find the number of valence electrons in the molecule. In $IF_{4}^{-}$, there are two atoms i.e., iodine and fluorine, and both of them are the elements belongs to group 17 so, they both have 7 electrons in their valence shell and there is one negative charge which means that there is the addition of one electron to total valence electron in the molecule. Therefore, the total number of valence electrons are:
$7+7+7+7+7+1=36$
There are four fluorine atoms attached to the iodine so, there are four covalent bonds and all the fluorine atoms will have three lone pairs. This constitutes 28 electrons and rest 4 electrons constitute the two lone pairs around the iodine atom. So, the coordination number of iodine will be 6 in which 4 are covalent bonds and 2 lone pairs. The shape will be a square planar in which all the corners are occupied by the fluorine atoms, one lone pair is above the plane, and one lone pair is below the plane. The structure is given below:

So, in square planar structure, the bond angle is ${{90}^{\circ }}$ therefore, bond angle in $IF_{4}^{-}$ will be ${{90}^{\circ }}$.
Note:
There is no repulsion between the lone pair and bond pair in the $IF_{4}^{-}$ because all the bond in $IF_{4}^{-}$ are in one plane but the lone pairs are above and below the planes so, there is no repulsion.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




