
pratyush took sulphur power on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in the figure.
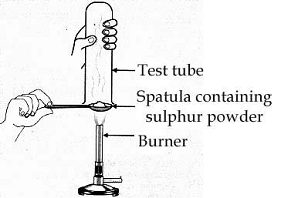
What will be the action of gas on dry litmus paper?

Answer
560.7k+ views
Hint: \[\text{S}{{\text{O}}_{\text{2}}}\] turns lime water into milky water.
Litmus paper is a coloured paper which is used to indicate the chemical nature of the solution.
Complete step by step answer:
In the classes of chemistry, we have carried out several common experiments in laboratories and let us now solve this question based on those concepts.
When sulphur powder is heated on spatula it forms sulphur dioxide. $\text{S}\,\text{+}\,{{\text{O}}_{\text{2}}}\,\,\xrightarrow{\text{heat}}\,\text{S}{{\text{O}}_{\text{2}}}$
- Oxides of sulphur are acidic in nature because they form acid on dissolving in water. \[\text{S}{{\text{O}}_{\text{2}}}\] is colourless gas with suffocating odour having the smell of burning sulphur?
\[\text{S}{{\text{O}}_{\text{2}}}\] is both oxidizing and reducing in nature, because in \[\text{S}{{\text{O}}_{\text{2}}}\] oxidation state of sulphur is +4 and it can either increase to +6 in (\[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]) or decrease to +2 in (\[{{\text{H}}_{\text{2}}}\text{S}\]). So, \[\text{S}{{\text{O}}_{\text{2}}}\] will be behind both oxidizing and reducing agents.
\[\text{S}{{\text{O}}_{\text{2}}}\,\text{+}\,\text{2}{{\text{H}}_{\text{2}}}{{\text{O}}_{(l)}}\,\,\to \,\,{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\,\text{+}\,\text{2}\,\text{ }\!\![\!\!\text{ H }\!\!]\!\!\text{ }\]
- Reducing nature of \[\text{S}{{\text{O}}_{\text{2}}}\] is due to the formation of nascent hydrogen. Moreover this nascent hydrogen is also responsible for the bleaching action of\[\text{S}{{\text{O}}_{\text{2}}}\].$\text{Coloured}\,\text{substance}\,\text{+}\,\text{ }\!\![\!\!\text{ H }\!\!]\!\!\text{ }\,\,\to \,\text{Decolourised}$
- The bleaching action of \[\text{S}{{\text{O}}_{\text{2}}}\] is temporary, as the decolourised substance is kept in open air; it gets oxidised and regain its previous colour. So, when the \[\text{S}{{\text{O}}_{\text{2}}}\] gas passes through dry litmus paper it will change the colour of litmus paper for a short period of time.
Note: When sulphur dioxide comes in the contact with blue litmus paper into red litmus paper this is because \[\text{S}{{\text{O}}_{\text{2}}}\] reacts with moisture, it will form sulphuric acid which will change the blue litmus paper into red litmus paper.
Litmus paper is a coloured paper which is used to indicate the chemical nature of the solution.
Complete step by step answer:
In the classes of chemistry, we have carried out several common experiments in laboratories and let us now solve this question based on those concepts.
When sulphur powder is heated on spatula it forms sulphur dioxide. $\text{S}\,\text{+}\,{{\text{O}}_{\text{2}}}\,\,\xrightarrow{\text{heat}}\,\text{S}{{\text{O}}_{\text{2}}}$
- Oxides of sulphur are acidic in nature because they form acid on dissolving in water. \[\text{S}{{\text{O}}_{\text{2}}}\] is colourless gas with suffocating odour having the smell of burning sulphur?
\[\text{S}{{\text{O}}_{\text{2}}}\] is both oxidizing and reducing in nature, because in \[\text{S}{{\text{O}}_{\text{2}}}\] oxidation state of sulphur is +4 and it can either increase to +6 in (\[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]) or decrease to +2 in (\[{{\text{H}}_{\text{2}}}\text{S}\]). So, \[\text{S}{{\text{O}}_{\text{2}}}\] will be behind both oxidizing and reducing agents.
\[\text{S}{{\text{O}}_{\text{2}}}\,\text{+}\,\text{2}{{\text{H}}_{\text{2}}}{{\text{O}}_{(l)}}\,\,\to \,\,{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\,\text{+}\,\text{2}\,\text{ }\!\![\!\!\text{ H }\!\!]\!\!\text{ }\]
- Reducing nature of \[\text{S}{{\text{O}}_{\text{2}}}\] is due to the formation of nascent hydrogen. Moreover this nascent hydrogen is also responsible for the bleaching action of\[\text{S}{{\text{O}}_{\text{2}}}\].$\text{Coloured}\,\text{substance}\,\text{+}\,\text{ }\!\![\!\!\text{ H }\!\!]\!\!\text{ }\,\,\to \,\text{Decolourised}$
- The bleaching action of \[\text{S}{{\text{O}}_{\text{2}}}\] is temporary, as the decolourised substance is kept in open air; it gets oxidised and regain its previous colour. So, when the \[\text{S}{{\text{O}}_{\text{2}}}\] gas passes through dry litmus paper it will change the colour of litmus paper for a short period of time.
Note: When sulphur dioxide comes in the contact with blue litmus paper into red litmus paper this is because \[\text{S}{{\text{O}}_{\text{2}}}\] reacts with moisture, it will form sulphuric acid which will change the blue litmus paper into red litmus paper.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




