
How many possible $ \angle FSeF $ bond angles are present in the $ Se{F_4} $ molecule?
Answer
481.2k+ views
Hint: Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom.
Complete Step By Step Answer:
A bond angle is the geometric angle between two adjacent bonds. Some common shapes of simple molecules include: Linear: In a linear model, atoms are connected in a straight line. The bond angles are set at $ {180^ \circ } $ .The bond angles depend on the number of lone electron pairs. The relationship between a bond's length and angle formed with an adjacent bond has been shown to be inverse; large bond angles result in shorter bond lengths.
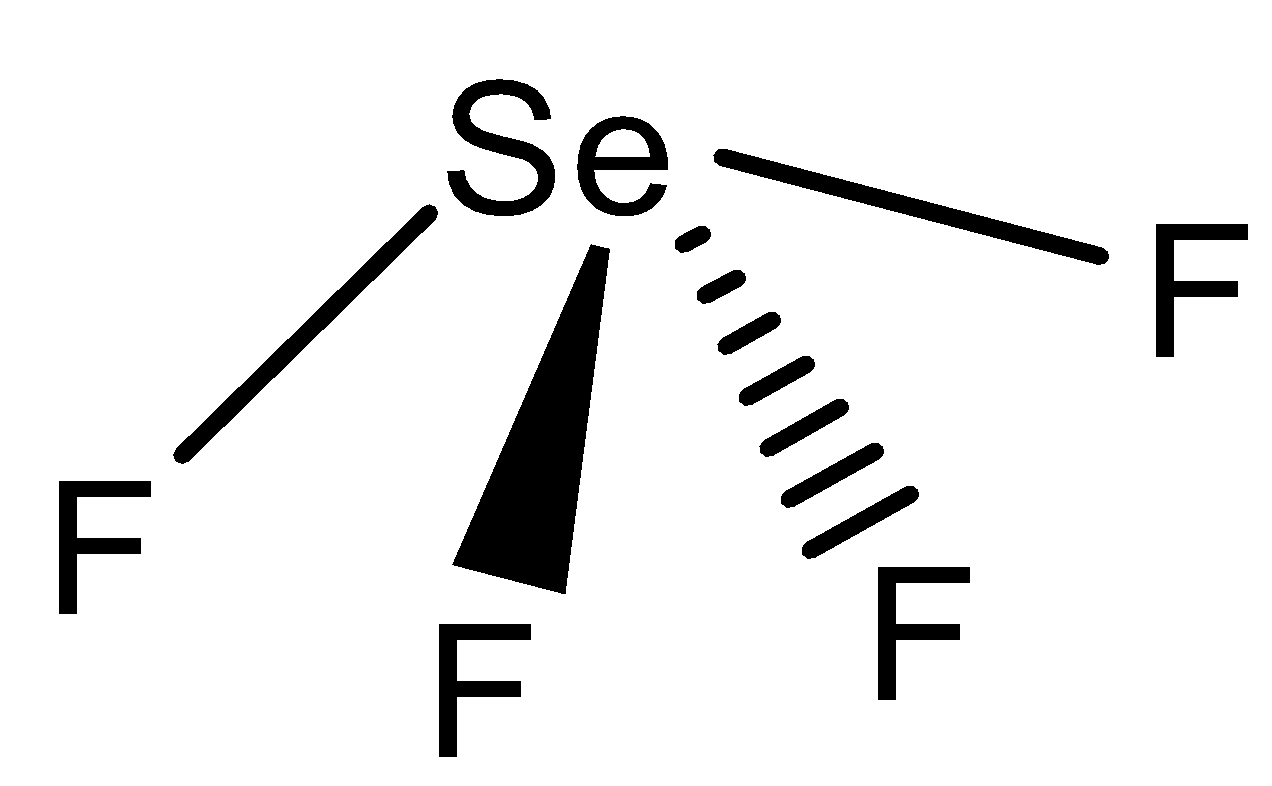
There are four $ F $ and one $ Se $ is present. There are $ 6 $ possible $ \angle FSeF $ bond angles are present in $ Se{F_4} $ molecule i.e $ \angle {F_1}S{F_2} $ , $ \angle {F_1}S{F_3} $ , $ {F_1}S{F_4} $ , $ {F_2}S{F_3} $ , $ {F_2}S{F_4} $ , $ \angle {F_3}S{F_4} $ .
Additional information:
Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism and biological activity. The angles between bonds that an atom forms depend only weakly on the rest of the molecule, i.e. they can be understood as approximately local and hence transferable properties.
Note:
Many factors lead to variations from the ideal bond angles of a molecular shape. Size of the atoms involved, presence of lone pairs, multiple bonds, large groups attached to the central atom, and the environment that the molecule is found in are all common factors to take into consideration.
Complete Step By Step Answer:
A bond angle is the geometric angle between two adjacent bonds. Some common shapes of simple molecules include: Linear: In a linear model, atoms are connected in a straight line. The bond angles are set at $ {180^ \circ } $ .The bond angles depend on the number of lone electron pairs. The relationship between a bond's length and angle formed with an adjacent bond has been shown to be inverse; large bond angles result in shorter bond lengths.

There are four $ F $ and one $ Se $ is present. There are $ 6 $ possible $ \angle FSeF $ bond angles are present in $ Se{F_4} $ molecule i.e $ \angle {F_1}S{F_2} $ , $ \angle {F_1}S{F_3} $ , $ {F_1}S{F_4} $ , $ {F_2}S{F_3} $ , $ {F_2}S{F_4} $ , $ \angle {F_3}S{F_4} $ .
Additional information:
Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism and biological activity. The angles between bonds that an atom forms depend only weakly on the rest of the molecule, i.e. they can be understood as approximately local and hence transferable properties.
Note:
Many factors lead to variations from the ideal bond angles of a molecular shape. Size of the atoms involved, presence of lone pairs, multiple bonds, large groups attached to the central atom, and the environment that the molecule is found in are all common factors to take into consideration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




